Introduction
Chromosomal polymorphisms (CPM) are minor variation of karyotype, found in >1% of the general population. Human chromosomes comprise of euchromatin and heterochromatin, the two functionally different parts of the genome.1 Euchromatin, the active portion of the genome has the important genes.2 Heterochromatin, the comparatively inactive part is of two types: constitutive, a stable form present in polymorphic variants; and facultative, a reversible form seen in the inactive X chromosome. Constitutive heterochromatin, formed by tandemly organized highly repeated sequences of satellite DNA, does not encode proteins. Previously, heteromorphisms did not appear to have any functional or phenotypic effect.3
Recent studies show that heterochromatin is not inert, but contains genes essential for viability, fertility and inheritance; Heterochromatin plays a role in spindle attachment and chromosome movement, meiotic pairing, and sister chromatid cohesion.4 Many couples with infertility and recurrent first trimester miscarriages had been extensively investigated but no specific cause for their challenged reproductive functions could be ascertained. We have tried to look for this unknown etiology through our study on chromosomal alterations in these couples.
Materials and Methods
The study population was selected from couples with recurrent pregnancy loss or primary infertility, attending JIMS hospital OPD, over a 1yr. period from May, 2021 to April, 2022. Inclusion criteria were recurrent miscarriages (>/=2) within 12 weeks of gestation and primary infertility of >1yr of unprotected intercourse. Couples with secondary infertility, intrauterine fetal death and thrombogenic or hormonal cause of recurrent pregnancy loss were excluded.
Karyotyping was carried out by 72-h culture of whole blood on 100 couples, meeting the inclusion criteria. Giemsa banding (GTG) was done in all cases. At least 20 metaphases were analysed.
The criteria used to score variants (qh+, ps+, pstk+) on autosomes was that the variant should be at least twice the size of its corresponding region on the other homologue in all the metaphases screened. The length of the Y chromosome heterochromatin (qh+/qh-) was similarly compared with that of chromosome 22. The percentage of chromosomal aberrations was calculated for both recurrent pregnancy loss and infertility cases.
Data collection was done as per institute ethics committee guidance, with informed consent.
Data analysis was done by percentage calculation, since the study sample size was limited.
Results
In our study, 56 couples had normal karyotype. 44 couples with RPL and infertility showed chromosomal abnormalities, which included chromosomal polymorphisms in 36 couples (81.81%), structural chromosomal abnormalities in 7 couples (15.90%). (Table 1, Figure 1)
Table 1
Showing chromosomal changes in our study
|
Chromosomal changes |
No. of couples |
|
Normal karyotype |
56 |
|
Polymorphisms |
36 |
|
Structural abnormalities |
7 |
|
Numerical abnormality 46,XX/47,XXX |
1 |
Chromosomal polymorphisms were found in chromosome 1 (1qh+) chromosome 9 (9qh+), the D, G chromosomes (13, 14, 15, 21 & 22) both in the satellite region and the stalk (ps+, pstk+). 12 couples showed Chr. Y polymorphisms (Yqh+ and Yqh-). (Table 2)
Chromosome 9qh+ was present in the majority of couples (33.33%). Among the D, G chromosomes, 8 (22.22%) couples showed polymorphism in chromosome 15 while only 1 couple (2.77%) showed polymorphism in chromosome 14 (Table 2).
Table 2
Showing various polymorphisms in our study
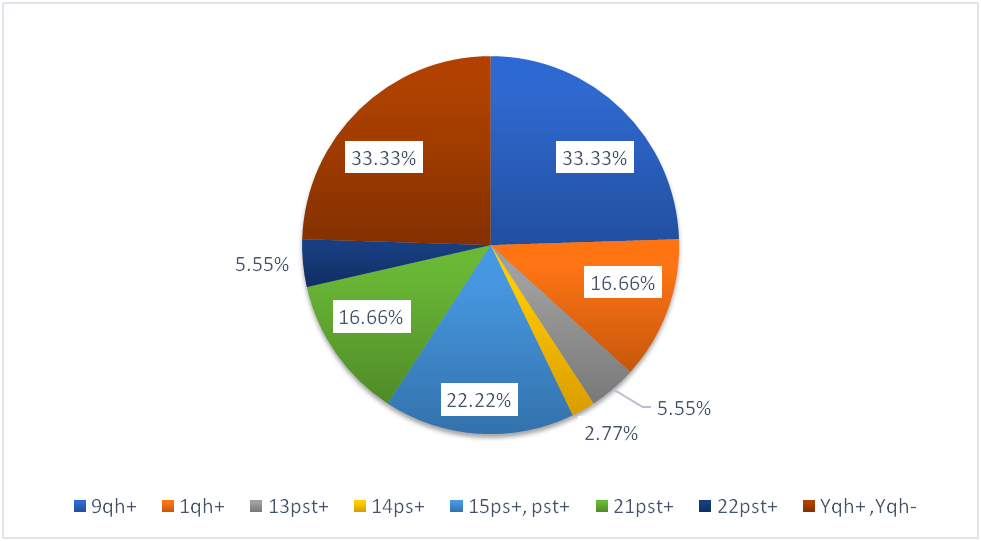
Our study showed a distribution pattern as follows- Polymorphism 9qh+ was found in 33.33% of our subjects, 15ps+ was found in 22.22% of our study population, 1qh+ was found in 16.66% of our couples. Among male partners, polymorphism in chr.Y (Yqh+ and Yqh-) was found in 33.33% of our study population. (Figure 2).
In our study, chromosomal polymorphism in Chr. 13 and 14 were found in females only; in Chr. 15, male and female distribution was 60% and 40% respectively. In chr. 21 and 22, there was equal representation in both sexes. (Figure 3)
Our study showed that 7 (16%) couples had structural chromosomal alterations, of which 5 were translocations (Reciprocal 4, Robertsonian 1), 1 was a deletion of chr.Y, and 1 was an inversion (pericentric) of chr. 9. One of the reciprocal translocation had an associated polymorphism of the Y chromosome. (Table 3, Figure 4)
Table 3
Structural chromosomal alterations and obstetric outcome
In our study 6 couples had polymorphism in both partners, and 5 couples had multiple polymorphisms in the same partner thus, multiple polymorphism was found in 11 couples and together they had 23 miscarriages. (Table 4). 25 couples had single polymorphism in one of the partners and collectively they had 54 miscarriages. On further analysis, we found average no. of RPLs in relation to single, double and triple polymorphisms per couple. However, the variation was not statistically significant. (Figure 5)
Table 4
Couples showing polymorphism in both partners, multiple polymorphisms in same partner
Table 5
No. of RPLs in case of multiple polymorphisms in same partner/both partner
|
No. of polymorphism/couple |
No. of RPL |
Mean ± SD |
P value = 0.5474 |
|
Single polymorphism |
25 |
2±1 |
|
|
Double polymorphism |
6 |
2± 1.0954 |
|
|
Triple polymorphism |
3 |
2.667±0.5774 |
Table 5 shows the no. of RPLs in case of single polymorphism and multiple polymorphisms in same couple. Using one-way Anova test, we tried to find relation between No. of polymorphism per couple and no. of RPLs, but found that it was not statistically significant (P value 0.5474).
Table 6
Average no. of RPLs in D,G chromosomes
|
Polymorphism |
Average RPL |
|
Chr.13ps+ |
1 |
|
Chr.14ps+ |
2 |
|
Chr.15ps+,pstk+ |
2.125 |
|
Chr.21ps+ |
1.66 |
|
Chr.22ps+ |
3 |
In our study, we analysed the obstetric outcome for couples with polymorphisms in D, G chromosomes (Chr. 13, 14, 15, 21, 22). The majority of RPLs were associated with chr. 22 polymorphism among the D, G chromosomes. (Table 6, Figure 6)
Discussion
Recurrent pregnancy loss or infertility is a devastating experience for couples and also a challenging problem for the obstetrician.
Our study shows the greater prevalence of polymorphism rather than structural or numerical chromosomal alterations in associated with RPl. (82% of couples with polymorphisms, 16% with structural and 2% with numerical chromosomal alterations).
Polymorphic variants are common in the q arms of paracentric heterochromatin of chromosomes 1, 9, 16, the acrocentric chromosomes (D, G chromosomes) (13.14.15, 21,22) and distal heterochromatin of the Y chromosome. (Figure 7).
Increased heterochromatin lengths are designated as qh+. Increase in length of the short arm satellites and stalks of the acrocentric chromosomes (13, 14, 15, 21 and 22) are designated, as 14ps+ and 13pstk+. Increase in length of the short arms are designated as p+ (e.g. 15p+).5 The short arms and satellites of acrocentric chromosomes also contain heterochromatin,6 while the stalks contain nucleolar organizing regions (NOR).7 The prevalence of 9qh+ and Yqh+ have been reported to be approximately 2.44% and 2.85%, respectively.8 In our study, multiple polymorphisms in 11 couples accounted for 23 RPLs, and single polymorphism in 26 couples accounted for 54 RPLs. Although polymorphisms of heterochromatic regions are considered as normal variants in humans, increased prevalence of chromosomal variants in infertile men have been found. Possibly, the large heterochromatic blocks (qh+) might destabilize the pairing of chromosomes and cause meiotic arrest, resulting in infertility.9 Our study revealed 12 males (24.48%) with chr.Yqh+/Yqh- polymorphisms, accounting for 15 RPLs. 2 of the couples showed primary infertility. These large heterochromatic blocks may cause meiotic arrest by deranged chromosomal pairing, thereby resulting in infertility.9
Chromosome 9
DNA sequence of human chromosome 9 recently revealed that it contains the largest autosomal block of heterochromatin, and is highly polymorphic in 6–8% of humans.10, 11 The maximum no. of polymorphisms in our study was found in Chr. 9 (33.33%), with females more than males. This was associated with 26 RPLs, again with more RPLs in females than in males;
Chromosome inversions
These are defined as the rearrangement produced by two break-points within the same chromosome, with the subsequent inversion and reinsertion of this fragment. Inversions are of two types - pericentric (if the inverted fragment includes the chromosome’s centromere) and paracentric (if the inverted fragment does not include the chromosome’s centromere).
A balanced pericentric inversion normally has no clinical consequences for its carrier and is found in 1–2% of general population. However, during meiosis there is a certain risk of inversion loop formation. An uneven number of crossing over events occuring within the inversion loop leads to de novo deletion, duplication or a combination of both in the offspring, producing unbalanced offspring.
Chromosome 9 inversion, is one of the most common structural balanced chromosomal variants, with an estimated incidence of about 3.5 percent. In some cases, it has been associated with congenital anomalies, growth retardation, infertility, recurrent pregnancy loss, and cancer. In our study 1 couple showed pericentric inversion in chr.9 with 2 RPLs. Šípek et al. compared inv (9) carriers and control subjects for each sex separately and found a statistically significantly higher incidence of heterochromatin variants in females, but not in males, with idiopathic reproductive failure.12
D/G chromosomes
D/G chromosomes commonly show increased heterochromatin in the q arm at the chromosome telomeres (qh+) and short variants at the stalk of the p arm containing NORs (pstlk+). The NORs contain ribosomal genes, which cluster on the short arm stalks, exhibiting polymorphic variations.13 For D/G chromosomes, the heterochromatin located in centromeres plays an important role in spindle attachment, chromosome movement, meiotic pairing, and sister chromatid cohesion.14 Females with D/G chromosome polymorphisms show a lower fertilization rate and cleavage rate. Increasing evidence has confirmed that female reproductive disorders are closely associated with chromosomal polymorphisms.15
However, D, G polymorphisms in males do not appear to adversely affect reproductive outcomes.15 Chromatin variations in D/G regions can cause defects in kinetochore assembly and centromere function, difficulty in homologous chromosome pairing, adversely affect cell division, and finally affect gamete formation. Our study portrayed greater no. of RPLs in chromosome 22, with an average of 3 RPLs. All the polymorphisms of chr13 & chr.14 were found in the females, equal distribution of polymorphisms in male and female were found in chr. 21, and chr. 22. Only chr. 15 polymorphisms were more in males than females. (Figure 3).
Structural chromosomal abnormalities
Chromosomal abnormalities have a major role in RPL and infertility.16 Numerical abnormality is associated with either the gain or loss of whole chromosomes. Structural abnormality have structural variations which includes translocations, inversions, insertions and deletions.
In our study, 7 couples (16%) showed structural abnormalities, with majority (72%) showing balanced translocation (4 reciprocal translocations and 1 Robertsonian translocaton). In reciprocal balanced translocations there is exchange of chromosomal fragments among different group other than acrocentric chromosomes. Robertsonian translocation shows translocation of q arms of acrocentric chromosomes, with loss of their p arms; total no. of chromosomes becomes 45. The translocated chromosomal segment length at breakpoints is important in reproductive failure.17 The breakpoint of balanced reciprocal translocation can disrupt important genes, resulting in RPL or infertility. Also, balanced translocation in parents can produce gametes with unbalanced gene dosage, causing spontaneous miscarriage of zygote in the first trimester or oogenic disturbances leading to unviable zygotes.18
One couple had deletion in q arm of Y chromosome, (46, Xdel(Y)(q11.2)) with primary infertility. AZFc gene, very important for male fertility, is located in this region. Deletion of this region in Chr. Y is the cause of infertility.
Numerical abnormality in mosaic form of 46, XX/47, XXX was found in 1 couple, with 2 RPLs. The extra Chr. X in some cells had caused increased gene dosage in those cells, leading to unviable zygotes. Extra sex chromosomes are commonly associated with recurrent miscarriages/infertility. An error in cell division called nondisjunction can result in reproductive cells with an abnormal number of chromosomes. 46, XX/47, XXX mosaicism is not inherited. It occurs as a random event during cell division in the early development of an embryo. Some of the cells of an affected person have two X chromosomes (46,XX), and other cells have three X chromosomes (47,XXX).
Conclusion
Our study showed that chromosomal polymorphisms were maximally found in Chr. 9. Among the D, G chromosomes, the maximum no. of polymorphisms were found in Chr.15, but Chr. 22 polymorphism (22ps+) was associated with the highest no. of RPLs. Average no. of RPLs in single polymorphism and double polymorphism per couple was same, but there was a slight increase in average no of RPLs in couples with 3 polymorphisms. However, it was not statistically significant.
The frequency of chromosomal abnormalities is found to be higher in cases with recurrent pregnancy loss and infertility, compared to the general population. Karyotyping the couple should be considered as part of reproductive management. Cytogenetic analysis gives important genetic information, thus acting as a good diagnostic tool, and helps to plan ART or perform prenatal testing, The latter resolves the trauma caused by pregnancy loss to the couple.