- Visibility 155 Views
- Downloads 57 Downloads
- Permissions
- DOI 10.18231/j.ijogr.2024.004
-
CrossMark
- Citation
Correlation between pre-operative platelets count and serum cancer antigen-125 level in epithelial ovarian carcer
Abstract
Background: Cancer antigen 125 (Ca-125) is currently used as an adjunct to ovarian cancer diagnosis, prognosis, and monitoring. Platelet (PLT) count and Ca-125 levels are both prognostic markers in ovarian cancer that are linked to inflammation and immune evasion.
Aim and Objectives: To determine the relationship between pre-operative platelet count and serum Ca-125 level, and their diagnostic accuracy for the prediction of stage IV epithelial ovarian cancer.
Materials and Methods: The study included forty-two women with histologic diagnosis of epithelial ovarian cancer managed at the University of Port Harcourt Teaching Hospital between January 1, 2016, and December 31, 2020. Following informed consent, a data collection form was used to obtain socio-demographic and clinical characteristics. Pretreatment levels of Cancer Antigen 125 (Ca-125) and Platelets count (PLT) were determined from blood samples. The Spearman correlation coefficient was used to determine the relationship between PLT and Ca-125, and the Receiver Operating Characteristic (ROC) curve analysis was used to assess the predictive accuracy of PLT count alone and PLT-Ca-125.
Results: The sample median platelet count was 308 (307) x 109/L and median Ca-125 was 286µ/ml (397). Pre-operative platelets count was significantly associated with Ca-125 (rho= 0.28 p-value = 0.03). Ca-125 had a statistically significant relationship with ovarian cancer histology (X2:19.522; p-value 0.001). PLT-Ca-125 (0.51) and PLT only (0.29) had a statistically significant positive correlation with ovarian cancer stage (p 0.001). Since it had an area under the curve (AUC) greater than 0.7, PLT-Ca-125 can be used as a predictive model to correctly stage patients with epithelial ovarian cancer. Ca-125 level (z:-2.24; p-value = 0.025) was significantly associated with thrombocytosis in ovarian cancer patients.
Conclusion: Platelet count and Ca-125 levels do correlate in blood samples taken from ovarian cancer patients prior to treatment. Furthermore, PLT-Ca-125 levels could be used to predict advanced stage disease.
Introduction
Ovarian cancer is the second leading cause of gynaecologic cancer deaths worldwide, trailing only cervical cancer.[1] It is the most fatal of all gynaecological cancers, with nearly two-thirds of patients diagnosed in the advanced stage. Ovarian cancer has a 5-year overall survival rate of around 48%. The prognosis varies depending on the stage, histologic type, and sensitivity to chemotherapy.[2] It is the leading cause of gynaecologic cancer deaths in most developed countries, with a lifetime risk of 1 in 70.[3] According to GLOBOCAN 2020, 313,959 new cases of ovarian cancer were diagnosed worldwide, with 207,252 deaths recorded; it is the eighth most common cancer in terms of incidence (6.6%) and mortality (4.2%) among females.[1]
Several studies in Nigeria have found a general trend towards an increase in the incidence of ovarian cancer. According to research from various centres in Nigeria, it accounts for 7%-26% of all gynaecological malignancies.[4]
Ovarian cancer is a diverse group of cancers. The most common type is epithelial ovarian cancer (EOC), which accounts for 90% of all cases.[5] EOC is most common in women aged 55 to 64 years, and deaths are most common in women aged 75 to 84 years. Currently, there is no available screening test for ovarian tumours. Although, Ca-125 and transvaginal ultrasonography are used for early detection, no reduction in ovarian cancer mortality rates has been observed.[6] Delays in diagnosis are caused by nonspecific symptoms and a lack of reliable biomarkers.[7] The treatment strategy for advanced EOC includes optimal cytoreductive surgery and chemotherapy. Approximately 70% of EOC patients are diagnosed with advanced Federation of Gynecology and Obstetrics (FIGO) stage III or even higher stage.[8]
Thrombocytosis (platelet count > 400,000/μL) has been linked to several cancers. In EOC, the rate of thrombocytosis ranges from 31 to 42%. [9] Many retrospective studies on EOC have identified thrombocytosis as a prognostic factor. [10] The increase in platelet count is caused by tumor-secreted cytokines such as interleukin (IL-6), which promotes megakaryocyte growth and thrombocytosis. [11] IL-6 is overproduced in a number of cancers and is associated with inflammation and immune suppression. [12] However, it is unclear whether the poor survival of thrombocytosis patients is caused by IL-6 or a result of IL-6-induced thrombocytosis. [13]
Thrombocytosis [platelet count > 400,000/μL] is associated with various cancers. The rate of thrombocytosis ranges from 31 to 42% in EOC.[9] Thrombocytosis is identified as a prognostic factor in many retrospective studies of EOC.[10] The increase of platelet count is due to tumour-secreted cytokines, such as interleukin (IL-6), which plays a role in stimulating the growth of megakaryocytes and thrombocytosis.[11] IL-6 is overproduced in a variety of malignancies and is related to inflammation and immune suppression.[12] However, it is still unclear whether the poor survival of patients with thrombocytosis is caused by IL-6 itself or is a result of IL-6-induced thrombocytosis.[13]
Many solid tumours, including colorectal cancer, breast cancer, pancreatic cancer, and lung cancer, have a poor prognosis when thrombocytosis is present before treatment.[14], [15], [16], [17], [18] Platelets may promote metastasis by improving tumour cell survival in the circulation system as well as extravasation and angiogenesis in target sites.[19] Furthermore, platelets promote cancer cell survival by preventing immune surveillance, stimulating angiogenesis, and arresting cancer cell cycles.[20] Tumour-derived IL-6 has the potential to increase hepatic thrombopoietin and stimulate the production of transforming growth factor (TGF)-β1, thereby activating TGF-β1/smad proliferation pathway in tumour cells. Pretreatment thrombocytosis is an independent adverse prognostic factor for cervical cancer, according to a recent systematic review and meta-analysis.[21] However, it is still controversial whether pretreatment thrombocytosis is an independent adverse prognostic factor for ovarian cancer.[22], [23]
Cancer antigen 125 (Ca-125) is a glycoprotein with a high molecular weight that is the most useful tumour marker for EOC because it indicates disease burden. Ca-125 levels are used in screening, diagnosis, monitoring of efficacy during chemotherapy, and follow-up management. The dynamic changes in Ca-125 levels at diagnosis and during chemotherapy were linked to drug and new agent chemosensitivity, tumour burden, and time of relapse. Furthermore, researchers observed that glycogen Ca-125 of tumour cells binds to natural killer (NK) cells and promotes immune evasion. Despite the fact that Ca-125 has been studied in EOC for decades, many unanswered questions remain.[24], [25]
Stage IV EOC is a systemic disease characterized by parenchymal metastases and extra abdominal organ metastases.[8] The poor survival of patients with thrombocytosis was thought to be caused by IL-6-induced thrombocytosis.[13] Platelet count and Ca-125 level are currently relevant in EOC.[25] However, the prognostic values of both markers were not compared. As a result, we set out to investigate the relationship between pre-operative platelet count and Ca-125, as well as the predictive value of combined PLT-Ca-125 in women with FIGO stage IV EOC.
Materials and Methods
Study area
This study was conducted at the gynaecological unit of the University of Port Harcourt Teaching Hospital (UPTH). The University of Port Harcourt Teaching Hospital is a 988-bed hospital in Alakahia, in Obio-Akpor Local Government Area of Rivers state. It is a tertiary hospital that serves as a referral centre for all levels of healthcare in Rivers state and other neighbouring states including Bayelsa, Imo and Abia. Every week, the gynaecology clinic is open from Monday to Friday, and each clinic session is led by a team of consultants. Patients are evaluated in the clinic before they are admitted into the gynaecogical ward for surgery.
Methods
This prospective observational study included 42 patients with histologically confirmed epithelial ovarian cancer who were managed at the University of Port Harcourt Teaching Hospital between January 1, 2018, and December 31, 2022. The purpose of the study was explained to the women, and an informed consent was obtained. An interviewer-administered data collection tool designed for this purpose was used to obtain the patients' information, and data was entered in a sequential order. Patients' baseline characteristics, The International Federation of Gynaecology and Obstetrics (FIGO) stage, complete blood count, serum cancer antigen 125 level, and histology were all included in the data. To ensure anonymity and ease of identification, each participant was given a distinct identity. The data collection tools were checked for accuracy and completeness on a daily basis. Patients with co-existing cancers, autoimmune disorders, known congenital thrombophilia, deep venous thrombosis, evidence of sepsis, anticoagulant therapy, and neo-adjuvant chemotherapy were excluded from this study.
Pre-treatment evaluation
All patients were subjected to physical, abdominal, and gynaecological pelvic examinations upon admission. Abdomino-pelvic MRI (Magnetic Resonance Imaging) and/or CT (Computed Tomography) were used to assess lymph node involvement and distant metastasis. Prior to the start of treatment, peripheral blood samples were collected and analyzed for absolute platelet count and serum Ca-125 levels. Thrombocytosis was defined as a platelet count greater than 400 x 109/L. The receiver operating characteristic (ROC) analysis was used to determine the cut-off value for PLT-Ca125.
Data analysis
The data was summarized using mean and standard deviation as appropriate. Spearman Rank correlation was used to assess the correlation between platelet counts, Ca-125 levels, and stage of ovarian cancer. ROC analysis with Area under the Curve (AUC) was used to determine the discriminative role and cut-off value of pretreatment platelet count and Ca-125. Sensitivity and specificity were determined using cut-off values. For statistical significance, the p-value was set at 0.05, and data was analyzed using SPSS version 25 at a 95% confidence interval.
Ethical consideration
The research and ethics committee of the University of Port Harcourt Teaching Hospital granted ethical approval for the study. Prior to their inclusion in the study, participants provided written informed consent. Personal identifying information was kept confidential.
Results
The study enlisted the participation of 42 patients. Most 17 (40.5%) were aged 45-54 years, with a mean age of 46.4 ± 10.6 years. Majority 29 (69.1%) were married, 25 (59.4%) had tertiary education, and 36 (85.7%) were still actively engaged in their respective occupations.([Table 1])
|
Attributes |
N (%) |
|
Age (years) |
|
|
≤24 |
1 (2.4) |
|
25-34 |
3 (7.1) |
|
35-44 |
13 (30.9) |
|
45-54 |
17 (40.5) |
|
55-64 |
6 (14.3) |
|
≥ 65 |
2 (4.8) |
|
Marital Status |
|
|
Single |
8 (19.0) |
|
Married |
29 (69.1) |
|
Divorced |
2 (4.8) |
|
Widowed |
3 (7.1) |
|
Education |
|
|
None |
2 (4.8) |
|
Primary |
5 (12.0) |
|
Secondary |
10 (23.8) |
|
Tertiary |
25 (59.4) |
|
Occupation |
|
|
#Technical/Associate Professional |
5 (12.0) |
|
$Professional |
13 (30.9) |
|
%Clerical Support |
1 (2.4) |
|
&Elementary |
3 (7.1) |
|
!Service/Sales Workers |
7 (16.7) |
|
>Skilled Workers/Farmers/Fishermen |
3 (7.1) |
|
<Craft/ Related Trade/Traders |
10 (23.8) |
|
Occupation |
|
|
Active |
36 (85.7) |
|
Inactive |
4 (9.5) |
|
Retired |
2 (4.8) |
|
Median Parity |
3 (4) |
|
Median No. of Living Children |
3 (4) |
|
Age at Menarche |
13 (1) |

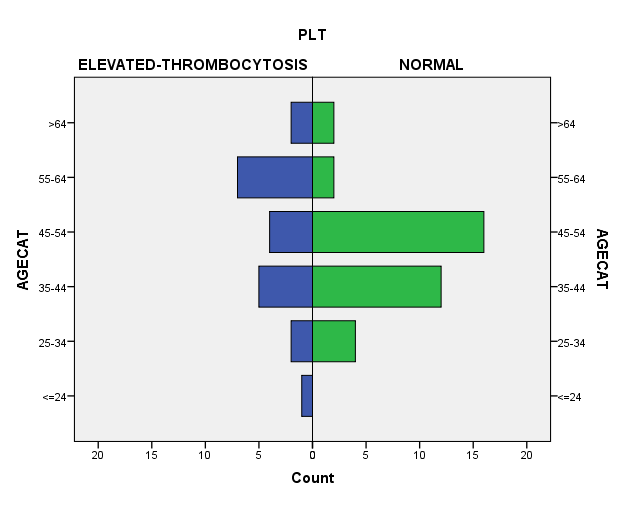
As shown in [Figure 1] and [Table 2], the prevalence of thrombocytosis (platelet count > 400 x 109/L) was 37%, with a median platelet count of 308 (307) x 109/L and a median Ca-125 level of 286/ml (397). [Figure 2] shows that thrombocytosis was more prevalent in the 55-64 age groups.
|
Variables |
Median (IQR) |
|
Median Platelet Count (109/L) |
308 (307) |
|
Median CA125 µ/ml |
286 (397) |
|
Median CA199 g/l |
9.8 (29.1) |
|
Median CEA g/l |
1 (92.8) |
|
Median AFP g/l |
3.6 (7.6) |
|
Mean LDH g/l |
285 ± 64.9 |
[Table 3] shows that in ovarian cancer patients, pre-operative platelet count was significantly associated with Ca-125 (rho= 0.28, p-value = 0.03). According to [Table 4], there was no significant statistical relationship between pre-operative platelet count (rho= 0.12 p-value = 0.38), Ca-125 (rho= 0.25 p-value = 0.07), and stage of ovarian cancer using both spearman correlation and Kruskal-Wallis test.
|
Variables |
rho |
p-value |
|
Platelet count vs. CA125 |
0.195 |
0.03* |
|
Variables |
rho |
p-value |
p-value@ |
|
Platelet count |
0.12 |
0.38 |
0.46 |
|
CA125 |
0.25 |
0.07 |
0.06 |
Bivariate analysis using the Kruskal-Wallis test reveals a statistically significant relationship between Ca-125 (X2: 19.522; p-value 0.001) and ovarian cancer histology, as shown in [Table 5]. [Table 6] shows that there was no statistically significant relationship between platelets count (X2: 0.944; p-value = 0.994) and ovarian cancer histology.
|
Histological Type |
Median Ca125 (µ/ml) |
X2 |
p-value |
|
Serous |
418.4 |
19.522 |
<0.001* |
|
Mucinous |
25.1 |
|
|
|
Endometroid |
375.5 |
|
|
|
Clear Cell |
218 |
|
|
|
Histology |
Median Ca125 (µ/ml) |
X2 |
p-value |
|
Serous |
315 |
0.994 |
0.911 |
|
Mucinous |
269 |
|
|
|
Endometroid |
427 |
|
|
|
Clear cell |
308 |
|
|
As shown in [Table 7], partial correlation was used to examine the relationship between platelet count alone, PLT-Ca-125, and ovarian cancer staging while controlling for age, age at menarche, and parity. PLT-Ca-125 (0.51) and platelet only (0.29) had a statistically significant positive correlation with ovarian cancer stage (p <0.001). These findings show that an increase in PLT-Ca-125 has a strong positive correlation with ovarian cancer stage. This implies that high PLT-Ca-125 levels are associated with late-stage ovarian cancer.
|
Parameters |
r |
p-value |
|
Platelet Only |
0.29 |
0.046 |
|
Platelet count-CA125 |
0.51 |
<0.001 |
In the study, predictive models of platelet counts only and PLT-Ca-125 as markers in ovarian cancer staging were performed using ROC curve analysis on platelet counts only and PLT-Ca-125 as an outcome. Figure 3 shows that PLT-Ca-125 can be used as a predictive model because it has an area under the curve (AUC) greater than 0.7. This means the model has a greater than 70% chance of correctly staging patients with epithelial ovarian cancer. [Table 8] shows that PLT-Ca-125 has a sensitivity of 73.3% and a specificity of 71.4% with a cut-off value of 0.224. Bivariate analysis using the Mann Whitney U test reveals a statistically significant relationship between Ca-125 (z:-2.24; p-value = 0.025) and thrombocytosis among the patients with ovarian cancer. This implies that the cases with thrombocytosis were significantly more likely to have advanced stage disease and elevated levels of pretreatment Ca-125 than those with normal platelet counts. This is shown in [Table 9].
|
Parameters |
AUC |
95% CI |
Cut-off |
Sensitivity % |
Specificity % |
p-value |
|
Platelet count only |
0.62 |
0.43-0.82 |
382.7 |
60 |
69 |
0.17 |
|
440.7 |
60 |
71.4 |
||||
|
Platelet count-CA125 |
0.77 |
0.59-0.94 |
0.224 |
73.3 |
71.4 |
0.003* |

|
Histology |
Median Ca125 (µ/ml) |
Z |
p-value |
|
Normal Platelet Count |
242 |
-2.24 |
0.025* |
|
Elevated Platelet Count |
421.7 |
|
|
Discussion
Despite new chemotherapeutic, surgical, and other auxiliary treatments, the 5-year survival rate in malignant ovarian tumours remains low. Thus, early detection and treatment are critical for extending the life and improving the quality of life of women with epithelial ovarian cancer. As a result, researchers are constantly looking for new markers for the early detection of malignant ovarian tumours.
In our study, the prevalence of thrombocytosis was 37%. This is comparable to the 31.0% observed by Stone et al.[9] but higher than the 22.3% and 13.8% reported by Allensworth et al.[26] and Feng et al.[27] respectively. It was, however, significantly lower than the 41.7% reported in Lagos.[28] This disparity could be explained by the fact that many of the women presented with advanced ovarian cancer.
We observed that thrombocytosis was associated with the tumour stage and histological type. These findings are consistent with several published studies involving patients with EOCs of any histological type, indicating that platelet counts increase concurrently with tumour progression and metastasis.[9], [26], [28], [29], [30], [31]
Ca-125 is a commonly used parameter in epithelial ovarian cancers for predicting advanced-stage disease, optimal debulking, and platinum resistance. Ca-125 levels are relatively low in early-stage serous ovarian cancers and rise as the tumour expands and burden increases. Ca-125 levels have been shown to increase proportionally with tumour stage and burden, and they are also known to rise in other organ pathologies like tubal pathologies, endometriosis, liver cirrhosis, inflammatory pelvic diseases, pregnancy, and so on.[10], [11]
The current study established an association between pretreatment platelet count and Ca-125 levels. This is consistent with reports from Poland,[22] Turkey,[32] Egypt,[33] and the United States,[23] which found that patients with thrombocytosis had higher Ca-125 levels, advanced stage disease, higher grade tumours, more frequent lymph node metastases, a larger volume of ascites, and shorter overall and disease-free survival. However, some previous studies found no evidence of a possible relationship.[34], [35]
Pretreatment platelet count and Ca-125 levels did not correlate with ovarian cancer stage, implying that they may not be useful in predicting outcome and overall survival. This is similar to a report from a Chinese university hospital.[36] This observation contradicts reports from Nigeria,[28] Korea,[37], [38] Turkey,[39] the United States,[9] and Brazil,[40] that reported a relationship between platelet count, Ca-125, and ovarian cancer stage, which was attributed to increased tumour burden. This suggests that platelet count and Ca-125 levels increase in tandem with tumour progression and metastasis.
These studies demonstrated that a platelet count and Ca-125 combination is a useful and easily accessible marker for predicting clinical outcomes and suggesting potential therapeutic strategies in patients with stage IV EOC. This disparity could be attributed to the larger sample size, study population, and the longer duration of the studies.
Our research found a significant statistical relationship between Ca-125 and the histological type of ovarian cancer. This implies that serous ovarian cancer had higher levels of Ca-125 than other histological types. Similar observations were made in Korea.[38] However, it contradicted the report from China which found no significant correlation.[36] Perhaps, the difference is due to the fact that the Chinese study was a retrospective study.
The study did not establish a correlation between pretreatment platelet count and histological type of ovarian cancer. This contradicts the report from Lagos and other published studies which showed a significant association between pretreatment thrombocytosis and histological type of ovarian cancer.[9], [26], [29], [30], [31] This observed difference may be due to the retrospective cohort study conducted by the researchers in Lagos.
In the study, predictive models were developed using ROC curve analysis on PLT alone and PLT-Ca-125 against stage IV ovarian cancer as an outcome. PLT-Ca-125 and tumour stage were found to have a strong positive correlation. This implies that, when compared to both markers alone, PLT-Ca-125 can be used to correctly stratify patients. As a result, it could be a useful marker in predicting advanced-stage disease. This finding is consistent with previous reports.[34], [41], [42]
The limitation of the study was the small sample size, and the patients were not followed up on to assess overall survival. In addition, because our centre is a foremost tertiary referral hospital in Rivers State, many ovarian cancer patients seen here have advanced disease, which may not be a fair representation of the general population. The fact that the study is a single-centre study with patients evaluated by the same team over time is an advantage. Furthermore, because this is a pilot study among women with epithelial ovarian cancer in Nigeria's southern region, the preliminary data collected will serve as the foundation for a larger future longitudinal study.
Source of Funding
No funding sources.
Conflict of Interest
The authors declare that they have no conflict of interests.
References
- Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 cancers in 185 countries. Ca Cancer J Clin. 2021;71(3):209-49. [Google Scholar]
- Bowtell D, Bohm S, Ahmed A, Aspuria P, Bast R, Beral V. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15(11):668-79. [Google Scholar]
- Siegel R, Miller K, Jemal A. Cancer statistics, 2015. Cancer J Clin. 2015;65:5-29. [Google Scholar] [Crossref]
- Okunade K, Okunola H, Okunowo A. A five-year review of ovarian cancer at a tertiary institution in Lagos, South-west. Niger J Gen Pract. 2016;14(2):23-7. [Google Scholar]
- Wentzensen N, Poole E, Trabert B, White E, Arslan A, Patel A. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016;34(24):2888-98. [Google Scholar]
- Siegel R, Miller K, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. [Google Scholar]
- Devan S, Pailoor J, Sthaneshwar P, Narayan V. Pattern of tissue expression of CA-125 and HE4 in primary epithelial ovarian tumours and correlation with serum CA-125 levels. Asian Pac J Cancer Prev. 2013;14(8):4545-8. [Google Scholar]
- Ataseven B, Chiva L, Harter P, Gonzalez-Martin A, Bois A. FIGO stage IV epithelial ovarian, fallopian tube and peritoneal cancer revisited. Gynecol Oncol. 2016;142(3):597-607. [Google Scholar]
- Stone R, Nick A, Mcneish I, Balkwill F, Han H, Bottsford-Miller J. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610-8. [Google Scholar]
- Kim S, Lee H, Go S, Lee S, Lee G. Clinical significance of the preoperative platelet count and platelet-to-lymphocyte ratio (PLT-PLR) in patients with surgically resected non-small cell lung cancer. Oncotarget. 2016;7(24):36198-206. [Google Scholar]
- Lin R, Afshar-Kharghan V, Schafer A. Paraneoplastic thrombocytosis: the secrets of tumor self-promotion. Blood. 2014;124(2):184-7. [Google Scholar]
- Chatterjee M, Osborne J, Bestetti G, Chang Y, Moore P. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science. 2002;298(5597):1432-5. [Google Scholar]
- Alberti C, Pinciroli P, Valeri B, Ferri R, Ditto A, Umezawa K. Ligand-dependent EGFR activation induces the co-expression of IL-6 and PAI-1 via the NFkB pathway in advanced-stage epithelial ovarian cancer. Oncogene. 2012;31(37):4139-49. [Google Scholar]
- Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Preoperative thrombocytosis is associated with survival after surgery for colorectal cancer. J Surg Oncol. 2012;106(7):887-91. [Google Scholar]
- Sasaki K, Kawai K, Tsuno N, Sunami E, Kitayama J. Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World J Surg. 2012;36(1):192-200. [Google Scholar]
- Stravodimou A, Voutsadakis I. Pretreatment thrombocytosis as a prognostic factor in metastatic breast cancer. Int J Breast Cancer. 2013;2013. [Google Scholar] [Crossref]
- Hwang S, Kim K, Cheong J, Kim H, An J, Hyung W. Impact of pretreatment thrombocytosis on blood-borne metastasis and prognosis of gastric cancer. Eur J Surg Oncol. 2012;38(7):562-7. [Google Scholar]
- Maráz A, Furák J, Varga Z, Kahán Z, Tiszlavicz L, Hideghéty K. Thrombocytosis has a negative prognostic value in lung cancer. Anticancer Res. 2013;33(4):1725-9. [Google Scholar]
- Gay L, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123-34. [Google Scholar]
- Davis A, Afshar-Kharghan V, Sood A. Platelet effects on ovarian cancer. Semin Oncol. 2014;41(3):378-84. [Google Scholar]
- Cheng J, Zeng Z, Ye Q, Zhang Y, Yan R, Liang C. The association of pretreatment thrombocytosis with prognosis and clinicopathological significance in cervical cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(11):24327-36. [Google Scholar]
- Friebe Z, Watrowski R, Bembnista M, Rokowska A, Włosiińska J, Dembińska M. Correlation between platelet count and CA-125 in ovarian cancer. Ginekol Pol. 2005;76(3):187-94. [Google Scholar]
- Li A, Madden A, Cass I, Leuchter R, Lagasse L, Karlan B. The prognostic significance of thrombocytosis in epithelial ovarian carcinoma. Gynecol Oncol. 2004;92(1):211-4. [Google Scholar]
- Rustin G, Hall M. Is CA125 useful in monitoring patients with platinum resistant ovarian cancer?. Ann Oncol. 2016;27(8):1365-6. [Google Scholar]
- Kim H, Choi H, Lee M, Suh D, Kim K, No J. Systemic inflammatory response markers and CA-125 levels in ovarian clear cell carcinoma: a two Center cohort study. Cancer Res Treat. 2016;48(1):250-8. [Google Scholar]
- Allensworth S, Langstraat C, Martin J, Lemens M, Mcgree M, Weaver A. Evaluating the prognostic significance of preoperative thrombocytosis in epithelial ovarian cancer. Gynecol Oncol. 2013;130(3):499-504. [Google Scholar]
- Feng Z, Wen H, Bi R, Duan Y, Yang W, Wu X. Thrombocytosis and hyperfibrinogenemia are predictive factors of clinical outcomes in high-grade serous ovarian cancer patients. BMC Cancer. 2016;16. [Google Scholar]
- Okunade K, Dawodu O, Adenekan M, Nwogu C, Awofeso O, Ugwu A. Prognostic impact of pretreatment thrombocytosis in epithelial ovarian cancer. Niger J Clin Pract. 2020;23(8):1141-7. [Google Scholar]
- Ma X, Wang Y, Sheng H, Tian W, Qi Z, Teng F. Prognostic significance of thrombocytosis, platelet parameters and aggregation rates in epithelial ovarian cancer. J Obstet Gynaecol Res. 2014;40(1):178-83. [Google Scholar]
- Qiu J, Yu Y, Fu Y, Ye F, Xie X, Lu W. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res. 2012;38(4):651-7. [Google Scholar]
- Polterauer S, Grimm C, Seebacher V, Concin N, Marth C, Tomovski C. Plasma fibrinogen levels and prognosis in patients with ovarian cancer: A multicenter study. Oncologist. 2009;14(10):979-85. [Google Scholar]
- Kuyumcuoglu U, Guzel A, Celik Y, Erdemoglu M. The association of preoperative thrombocytosis with prognostic factors in malign ovarian tumor. Eur J Gynecol Oncol. 2010;31(5):514-6. [Google Scholar]
- Agameya A, Labib K, Moiety F. Using platelet-to-lymphocyte ratio as a diagnostic marker in malignant ovarian tumors. Int J Reprod Contracept Obstet Gynecol. 2018;7(6):2089-92. [Google Scholar]
- Chen J, Huang Q, Wan T, Tu H, Gu H, Cao J. Combined score of pretreatment platelet count and CA125 level (PLT-CA125) stratified prognosis in patients with FIGO stage IV epithelial ovarian cancer. J Ovarian Res. 2019;12(1). [Google Scholar] [Crossref]
- Baert T, Camp JV, Vanbrabant L, Busschaert P, Laenen A, Han S. Influence of CA125, platelet count and neutrophil to lymphocyte ratio on the immune system of ovarian cancer patients. Gynecol Oncol. 2018;150(1):31-7. [Google Scholar]
- Hu Q, Hada A, Han L. Platelet count as a biomarker for monitoring treatment response and disease recurrence in recurrent epithelial ovarian cancer. J Ovarian Res. 2020;13(78). [Google Scholar] [Crossref]
- Oh J, Choi M, Park H, Lee C, Lee J, Chong S. Preoperative Thrombocytosis Is an Independent Poor Prognostic Factor in Patients with Epithelial Ovarian Cancer. Clin Exp Thromb Hemost. 2014;1(1):17-21. [Google Scholar]
- Eo W, Kim K, Park E, Kim H, Kim H, Koh S. Diagnostic accuracy of inflammatory markers for distinguishing malignant and benign ovarian masses. J Cancer. 2018;9(7):1165-72. [Google Scholar]
- Kurban Y, Aslan F. Association between Stage and Degree of Differentiation with Ca-125 Levels and Inflammatory Response Markers in Malignant Serous Tumors of the Ovary. J Androl Gynaecol. 2021;9(1):1-4. [Google Scholar]
- Nomelini R, Oliveira L, Tavares-Murta B, Murta E. Parameters of blood count and tumor markers: a retrospective analysis and relation to prognostic factors in ovarian cancer. Eur J Gynaecol Oncol. 2017;38(3):364-7. [Google Scholar]
- Miran N. Thrombocytosis and CA125 as Predictor of Malignancy in Gynaecological Pelvic Mass. J Fac Med. 2017;59(3):239-43. [Google Scholar]
- Yavuzcan A, Caglar M, Ozgu E, Ustun Y, Dilbaz S, Ozdemir I. Should Cut-Off Values of the Risk of Malignancy Index be Changed for Evaluation of Adnexal Masses in Asian and Pacific Populations?. Asian Pac J Cancer Prev. 2013;14(9):5455-9. [Google Scholar]
How to Cite This Article
Vancouver
Alegbeleye JO, John CO. Correlation between pre-operative platelets count and serum cancer antigen-125 level in epithelial ovarian carcer [Internet]. Indian J Obstet Gynecol Res. 2024 [cited 2025 Sep 15];11(1):17-23. Available from: https://doi.org/10.18231/j.ijogr.2024.004
APA
Alegbeleye, J. O., John, C. O. (2024). Correlation between pre-operative platelets count and serum cancer antigen-125 level in epithelial ovarian carcer. Indian J Obstet Gynecol Res, 11(1), 17-23. https://doi.org/10.18231/j.ijogr.2024.004
MLA
Alegbeleye, Justina Omoikhefe, John, Celestine Osita. "Correlation between pre-operative platelets count and serum cancer antigen-125 level in epithelial ovarian carcer." Indian J Obstet Gynecol Res, vol. 11, no. 1, 2024, pp. 17-23. https://doi.org/10.18231/j.ijogr.2024.004
Chicago
Alegbeleye, J. O., John, C. O.. "Correlation between pre-operative platelets count and serum cancer antigen-125 level in epithelial ovarian carcer." Indian J Obstet Gynecol Res 11, no. 1 (2024): 17-23. https://doi.org/10.18231/j.ijogr.2024.004
