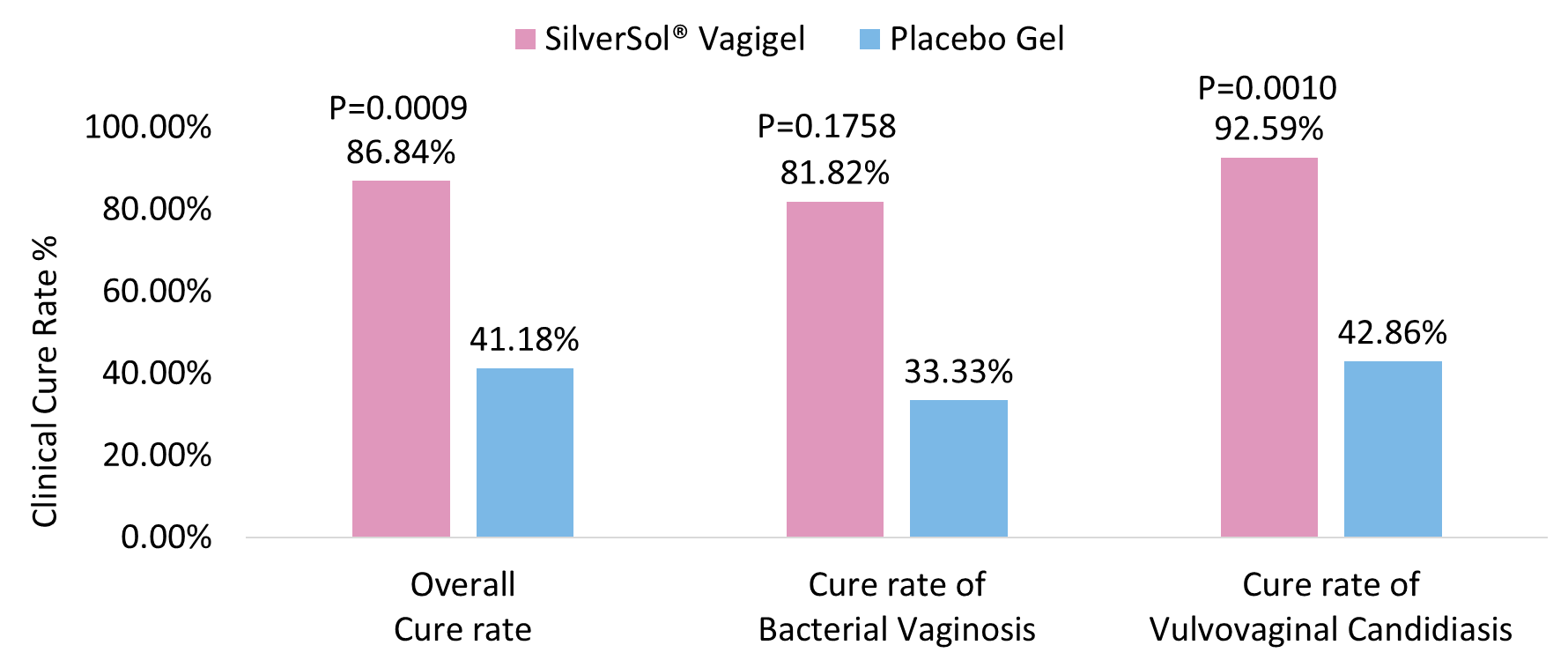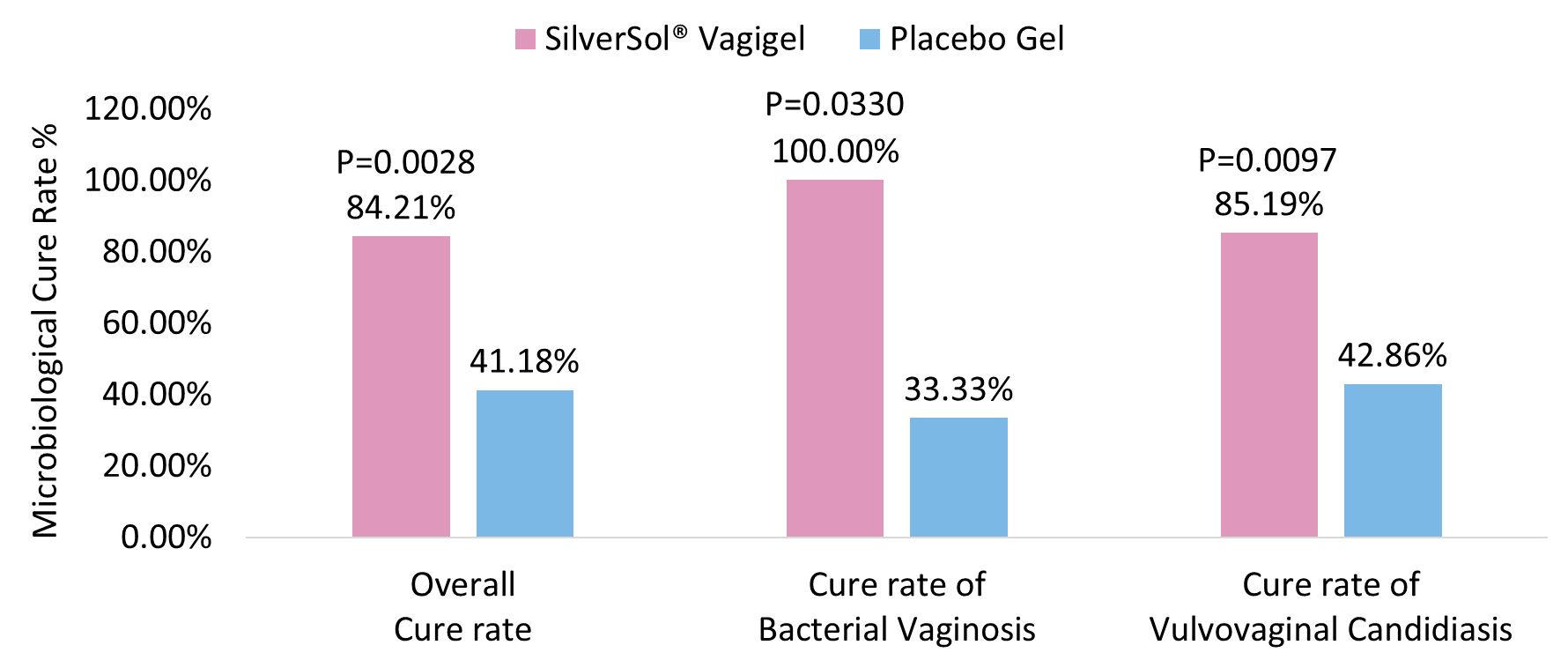Introduction
Vaginal infections, often termed "Infectious Vaginitis," are common concerns for women across the lifespan, with a higher prevalence among women of reproductive age. It is the most frequent reason for women to seek medical attention.1, 2 Although these infections are not associated with significant mortality, they are often associated with high levels of anxiety and have a negative impact on sexual life, self-esteem, and quality of life.1
The more prevalent vaginal infections are caused by disturbances in the natural vaginal microbiome. The normal vagina flora is characterized by the dominance of lactic acid-producing bacteria, especially Lactobacillus spp., which help to maintain an acidic pH of vaginal fluids within the range of 3.5-4.5. Along with lactobacilli, a healthy, acidic premenopausal vagina also contains a heterogeneous mixture of Gardnerella vaginalis, Escherichia coli, group B streptococcus (GBS), genital Mycoplasma species, Candida albicans, and other species.1, 2
The most commonly documented cause of vaginitis is bacterial vaginosis (BV), reported in 22 to 50% of cases. BV is characterized by a dramatic switch of vaginal bacterial flora from normal predominant Lactobacilli to a polymicrobial flora.2, 3 Vulvovaginal candidiasis (VVC) is the second most common vaginal infection reported in 17% to 39% of cases, while trichomoniasis, caused by Trichomonas vaginalis, is the most common non-viral sexually transmitted infection reported in 4 to 35% of cases.3, 4 True mixed infection rarely occurs in women with vaginitis, but coinfection occurs much more frequently. Coinfection with Candida species is observed in about 20%-30% of women with BV. The coinfection rates of BV pathogens and trichomoniasis are even more prevalent, ranging between 60% to 80%.5 Other etiologies of vaginitis include vulvar skin diseases, desquamative inflammatory vaginitis, and genitourinary syndrome of menopause.2, 3
Despite the availability of numerous oral, topical, and intravaginal medications for the treatment of vaginal infections, their management remains challenging. Associated side effects, development of resistance, lack of prevention and destruction of biofilms, and higher recurrences are the limitations of currently available conventional therapies. Ineffectiveness due to poor penetration and rapid removal from the vaginal canal are the major hurdles in achieving infection cure with local antimicrobial therapies.6 There is a need to develop newer therapeutic options for treating vaginal infections that overcomes the limitations of current therapies.
During the last decades, nanotechnologies-based formulations, such as nanoparticles, have been explored to overcome the limitations of current therapies for vaginal infections. These novel formulations can improve local drug delivery, biodistribution, retention, and uptake in vulvovaginal tissues. Other important benefits nanotechnology-based formulations offer are reduced toxicity, enhanced patient compliance, and improved treatment outcomes.6
This study evaluated the efficacy and safety of Colloidal Nanosilver Gel (SilverSol® Vagigel) composed of 32 ppm SilverSol® (a patented colloidal nano silver technology from American Biotech Labs, USA) in combination with 0.2% lactic acid in the treatment of BV and VVC. SilverSol® Vagigel is manufactured by Viridis BioPharma Pvt. Ltd., Mumbai.
Materials and Methods
Study design
This was a multicenter, randomized, double-blind, placebo-controlled pilot study. The study was conducted at two Investigator’s Sites, Titan Hospital Plaza, Thane; Akshay Surgical and Maternity Home, Thane between 07th June 2022 to 29th November 2022.The Independent Ethics Committee approved the study protocol (Protocol number: BCR-VIR-001; Dated: 09 Nov 2021). The IEC also approved the informed consent document (ICD) and. Drug dosing card. The current study was registered in Clinical Trials Registry- India (CTRI registration no: CTRI/2022/03/041393 Dated: 25/03/2022). The study was conducted as per the International Council for Harmonization (ICH) guidelines for Good Clinical Practice (E6R2), new drug and clinical trials rules 2019, the declaration of Helsinki (Brazil, 2013), the Indian Council of medical research (ICMR) guidelines (2017) for biomedical research on human subjects, and in accordance with other applicable guidelines.
All the participants were informed about the details of the study procedure during the screening visit. All patients provided written consent to participate in the study before being screening.
Participants
Post-menarchal female subjects between the age of 18 to 65 years with clinical diagnosis of BV and VVC were enrolled in the study. The clinical diagnosis of BV was confirmed based on the presence of all four Amsel criteria (Homogeneous vaginal discharge (off-white, milky or gray, thin), positive KOH whiff test, vaginal secretions pH of > 4.5, clue cells > /= 20 percent of vaginal squamous epithelial cells on saline "wet mount") and diagnosis of VVC was confirmed with presence of yeast forms (hyphae or pseudo hyphae) or budding yeasts on the KOH or saline preparation from the inflamed vaginal mucosa or secretions with two or more signs and symptoms of VVC and Vaginal pH less than or equal to 4. 5 (Signs: edema, redness, or excoriation. Symptoms: itching, burning, and/or irritation). Subjects were refrained from using intravaginal products throughout the study (e.g., douches, feminine deodorant sprays, Spermicides, tampons, and diaphragms).
Subjects with other infectious causes of vulvovaginitis, like Chlamydia trachomatis, Neisseria gonorrhoeae, and Herpes simplex, were excluded from the study. Other key exclusion criteria were the presence of any condition or illness, including vulvar and vaginal conditions, that, in the opinion of the investigator, would preclude accurate evaluation of the subject's condition and/or confound the interpretation of the subject's treatment response and history of cervical intra-epithelial neoplasia (CIN) or cervical carcinoma. Subjects using systemic (oral, intravenous [IV], or intramuscular [IM]), intravaginal/ vulvovaginal antifungal, antimicrobial, or corticosteroid therapy within 14 days of participation in the study and currently on any anticoagulation therapy or immunosuppressive therapy were excluded. The subjects were not included if they were pregnant, lactating, or planning a pregnancy during the study period.
Treatment
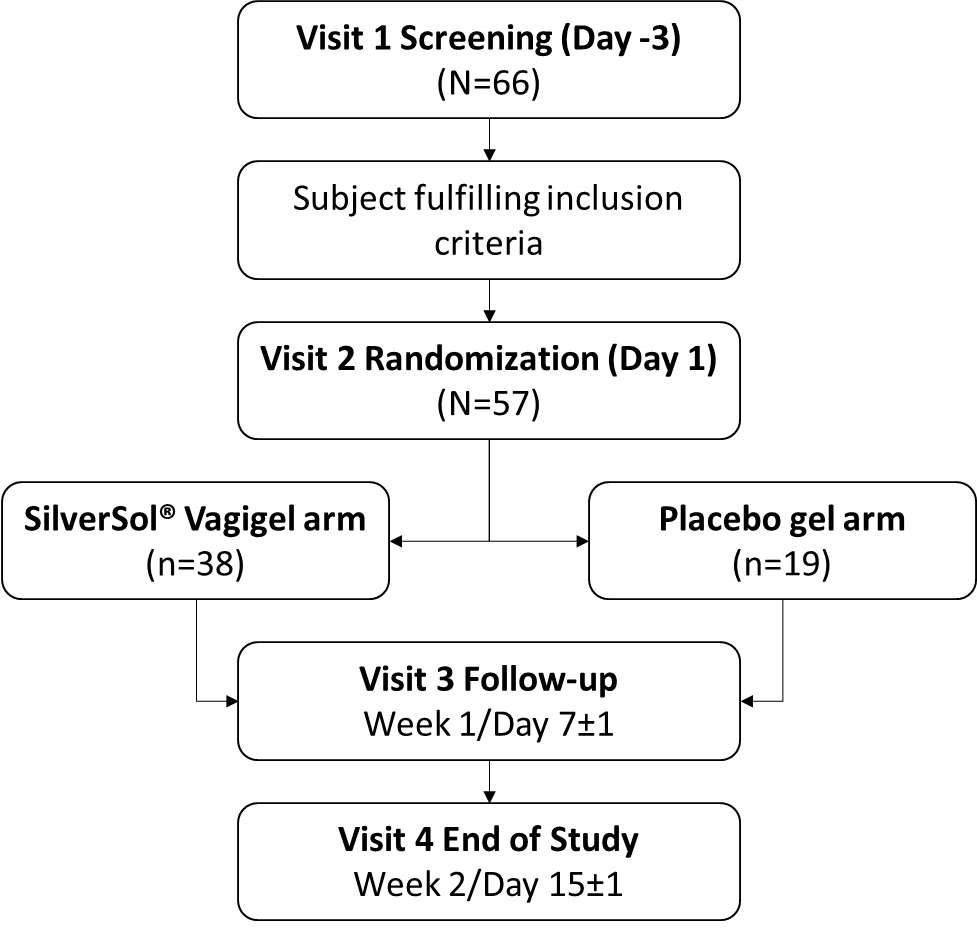
A total of 66 subjects were screened, of which 57 subjects were randomized in the study. Subjects were randomized in ratio 2:1 in one of the two arms to receive treatment with SilverSol® Vagigel (n=38) or placebo (vehicle) gel (n=19). The dose was one 4 gm application inserted into the vagina with an applicator device, once daily at bedtime for internal use and 2 gm gel for local application to the vulva and vagina for external use twice daily. The study treatment period was 14 days. The follow-up was conducted at week 1/Day 7±1, and the end of the study visit was at week 2/Day 15±1.
Primary and secondary endpoints
The primary endpoint evaluated was the proportion of subjects achieving clinical cure at the end of the study visit. Clinical cure for bacterial vaginosis was defined as resolution of the abnormal vaginal discharge, a negative whiff test and the presence of clue cells at less than 20% of the total epithelial cells on microscopic examination of the saline wet mount. For vulvovaginal candidiasis, the clinical cure rate was defined as the absence of all signs and symptoms of VVC.
The secondary endpoint evaluated was the proportion of subjects achieving microbiological cure at the end of the study visit. The microbiological cure for bacterial vaginosis was defined as a Nugent score of less than 4. Microbiological cure for vulvovaginal candidiasis was defined as the absence of yeast forms (hyphae or pseudo hyphae) or budding yeasts on the KOH or saline preparation, and that for trichomoniasis it was defined as negative wet mount assessment for T vaginalis.
Results
Demographics and other baseline characteristics
A total of 57 female subjects were randomized in the study, and all of them completed the study. The demographic pattern of the participant is given in Table 1.
Table 1
Summary of demographic and disease characteristics of the subjects at the baseline
There were 14 randomized subjects with bacterial vaginosis, of which 11 (28.95%) were randomized in the SilverSol® Vagigel arm and 03 (15.79%) in the placebo gel arm. There were 43 randomized subjects with vulvovaginal candidiasis, of which 27 (71.05%) were randomized in the SilverSol® Vagigel arm, and 16 (84.21%) were randomized in the placebo gel arm. There were no subjects enrolled with diagnosis of trichomoniasis.
Primary endpoints
Overall clinical cure
The proportion of female subjects achieving clinical cure of overall vaginal infections was significantly higher in the SilverSol® Vagigel arm than in the placebo gel arm (P=0.0009; Figure 2).
Clinical cure of bacterial vaginosis
The proportion of female subjects achieving clinical cure of bacterial vaginosis was higher in the SilverSol® Vagigel arm than in the placebo gel arm. (P=0.1758; Figure 2).
Clinical cure of vulvovaginal candidiasis
The proportion of female subjects achieving clinical cure of vulvovaginal candidiasis was also significantly higher in SilverSol® Vagigel than those treated with placebo gel (P= 0.0010; Figure 2).
Secondary endpoints
Overall microbiological cure
The microbiological cure rate for overall vaginal infections was significantly higher after treatment with SilverSol® Vagigel than with placebo gel (P=0.0028; Figure 3).
Microbiological cure for bacterial vaginosis
The microbiological cure rate of bacterial vaginosis was significantly higher in SilverSol® Vagigel arm compared to placebo gel arm (P=0.0330; Figure 3).
Microbiological cure of vulvovaginal candidiasis
The microbiological cure rate of vulvovaginal candidiasis was significantly higher in SilverSol® Vagigel arm compared to placebo gel arm (P=0.0097; Figure 3).
Discussion
Despite the high prevalence of vaginal infections, the available therapeutic options are predominantly conventional, presenting limitations in terms of efficacy and safety.1 Use of oral antimicrobials in the treatment of vaginal infections is primarily limited by associated side effects, development of resistance and higher recurrences, while ineffectiveness due to poor penetration and rapid removal from the vaginal canal are limiting factors for conventional vaginal antimicrobial therapies.6 The azole-based oral antimicrobial therapies are associated with a wide range of side effects, gastrointestinal effects (metallic taste in the mouth, nausea, vomiting), adverse hepatic effects, drug interactions, interference with the central nervous system, sex and thyroid hormones, and testosterone biosynthesis. They are also reported to have an increased risk of candida infection.7, 8
The presence of a thick vaginal multi-species biofilm makes the treatment of vaginal infections, especially BV and VVC, more challenging. Biofilms formed by G. vaginalis and Candida spp., the key pathogens in BV and VVC, respectively, contribute to potentially important virulence attributes and mechanisms of resistance often encountered clinically.9 When antimicrobials do not effectively eliminate biofilms, biofilm-related infections tend to persist and have a high rate of relapse and recurrence.10 Metronidazole and recent alternative tinidazole are the mainstay therapy for treating BV. However, these standard antibiotics cannot fully eradicate the vaginal biofilm, resulting in BV recurrence in more than half of the patients receiving guideline-recommended treatments. The resurgence of the BV biofilm after treatment cessation and failure to reestablish an optimal vaginal microbiome after treatment are the reasons for the high BV recurrence.10, 11 Clotrimazole is the first-line treatment for VVC, with fluconazole reserved for cases not responding to clotrimazole treatment. However, recently a very high level of clotrimazole resistance among the C. albicans isolates has been documented.12 Clotrimazole is typically the first-line treatment for vulvovaginal candidiasis, with fluconazole being reserved for cases with no response to clotrimazole treatment. Recently a very high label of Clotrimazole resistance among the C. albicans isolates has been documented.12 Higher rates of fluconazole-resistant VVC are also reported in many studies.13, 14 Trichomoniasis can be treated with either metronidazole or tinidazole. However, antimicrobial resistance is an emerging concern with high rates of recurrent trichomoniasis cases. It has also been observed that metronidazole gel is ineffective in more than 50% of trichomoniasis cases.15
In search of newer formulations to reduce adverse effects and to contrast microbial resistance and infection recurrences, researchers are developing vaginal systems for the local delivery of antimicrobials. Among all, nanosystems, such as silver nanoparticles, could be exploited to improve the treatment outcomes in vaginal infections by overcoming the limitations of current therapies.6 Silver nanoparticles are known for their excellent antibacterial activity that covers Gram-negative and Gram-positive bacteria, including multidrug-resistant strains.16 Silver nanoparticles have shown antibacterial action against vaginal pathogens, including Pseudomonas aeruginosa.17 Silver nanoparticles also exhibited antibacterial activity against G. vaginalis in an in-vitro study.18 Silver nanoparticles have demonstrated potent in-vitro antifungal activity against the resistant C. isolates.19 When combined with fluconazole, silver nanoparticles offer a synergistic effect against fluconazole-resistant C. albicans. It is a promising strategy for treating fluconazole-resistant fungal infections, including vulvovaginal candidiasis.20
Several studies have also demonstrated the anti-biofilm activity of colloidal nanosilver against Gram-positive and Gram-negative bacteria, and also C. albicans, which effectively prevents biofilm formation and eradicates bacteria within established biofilms.21 In an in-vitro study, almost complete prevention of biofilm formation by E. coli, S. aureus, and C. albicans, and more than 50% inhibition in the case of Enterococcus sp., coagulase-negative staphylococci, and P. aeruginosa was observed after 72 hours of incubation with silver nanoparticles.22 In another in-vitro study, silver nanoparticles exhibited potential anti-biofilm activity against the biofilm formed by P. aeruginosa and Staphylococcus epidermidis with staggering 95% inhibition of biofilm formation within 24 hours. 23 Another in-vivo study on the mouse wound model demonstrated that SilverSol® gel inhibits the growth of S. aureus, P. aeruginosa, A. baumannii, and two different methicillin-resistant S. aureus (MRSA) strains. The results showed the absence of viable cells in mice treated with colloidal silver gel, while the biofilm was detected in control mice. 24 Colloidal silver is a potent inhibitor of C. albicans biofilm formation. Silver nanoparticles disrupt the fungal cell wall and achieve their anti-biofilm effect by infiltrating it.25, 26, 27 The current evidence suggests that colloidal nanosilver may serve as a promising intervention for the prevention and treatment of biofilm-associated vaginal infections, such as BV and VVC.28, 29
Colloidal nanosilver has potent antimicrobial30 properties even at low concentrations and impressive anti-inflammatory properties. The safety of Colloidal nanosilver with the topical application and human consumption has been demonstrated in previous studies.31, 32 Studies have also demonstrated the wound-healing properties of Colloidal nanosilver. In a recently conducted study, it has been shown that SilverSol® can exert potent activity against different species of parasitic helminths.33 The SilverSol® gel was effective in preventing biofilm formation by S. mutans, S. sanguis, and S. salivarius.34 In an in vitro study, the SilverSol® gel in combination with Betadine antiseptic solution was proven to be effective in inhibiting the growth of bacterial biofilms.35 In several clinical studies, the SilverSol® has shown to be effective in oro-dental conditions including periodontitis and gingivitis,36 tinea infection and intertrigo37, 38 and in post-aesthetic skin procedures.37, 39
Colloidal nanosilver effectively inhibits the growth of various pathogens; however, it does not disturb the normal vaginal flora or affect commensal lactobacilli in the vagina. Cell surface barrier action of lactobacilli,40 robust metal cation efflux transporter activity of lactobacilli41 and lack of heme and functional electron transport chain (ETC) in lactobacilli42 spare it from the antimicrobial activity of nanosilver particles.
SilverSol® is considered a highly effective and broad-spectrum antimicrobial agent. It has demonstrated the ability to rapidly and completely eliminate bacteria, with a kill rate of 99.99900%. Notably, SilverSol® has the advantage of not causing bacterial resistance, a concern associated with many other antibiotics. This unique characteristic prevents bacteria from developing mutations to evade its activity.43
In-vitro evaluation of the antimicrobial activity of SilverSol® against vaginal pathogens demonstrated that as long as the silver concentration is sufficient to inhibit and kill any bacteria present in the vaginal mucosa, topical silver would be superior to that of the narrow-spectrum topical antifungal and antibacterial preparations. Evaluating the MIC (Minimum Inhibitory Concentration) of SilverSol® against major vaginal pathogens such as C. albicans, P. aeruginosa, and T. vaginalis showed MIC values comparable to the standards tested. In the kill-time study, SilverSol® quickly and completely destroys all the yeasts (C. albicans) and bacteria (Staph. aureus, MRSA, P. aeruginosa, vancomycin-resistant Enterococci (VRE), and E. coli) within one hour. SilverSol® showed antimicrobial activity against a broad spectrum of disease-causing pathogens including numerous bacterial and fungal strains (Staph. aureus, MRSA, P. aeruginosa, E. coli, and C. albicans) in the presence of vaginal fluid (simulant). SilverSol® with simulant completely killed the bacteria and fungi at 5 log reduction, i.e., 99.999% within one hour of contact time. In-vivo evaluation of the antimicrobial activity of SilverSol® against vaginal pathogens in an experimental mouse model of vaginitis demonstrated antibacterial activity against P. aeruginosa and antifungal activity against C. albicans. SilverSol® significantly reduced bacterial load compared to Ciprofloxacin, fungal burden compared to clotrimazole, and parasite load compared to metronidazole. An in-vivo study evaluating the effect of SilverSol® in an experimental mouse model of vaginitis showed no impact on the growth of Lactobacillus acidophilus in the vagina. The study concluded that SilverSol® could be safely applied intravaginally without harming the normal vaginal Lactobacillus flora. (Data on file, Viridis BioPharma.)
The published literature and the outcomes of the in-vitro and in-vivo preliminary studies provided the rationale for the clinical evaluation of the use of SilverSol® Vagigel in human vaginal infections. In the current study, the overall clinical and microbiological cure ratein BV and VVC was significantly higher with SilverSol® Vagigel when treated for 14 days. A complete microbial cure (100%) was reported in female subjects with BV, associated with clinical cure rate of 81.82%. In subjects with VVC, the clinical cure rate was achieved in 92.59%, correlated with (85.19%) of the microbiological cure rate.
It has been known that lactic acid produced by Lactobacillus spp. in vaginal flora lowers and maintains the vaginal pH in the range of 3.8-4.5. Lactic acid is the key to maintaining a medium that favors lactobacilli growth in a vaginal environment. Lactic acid production is a hallmark of a normal vaginal microbiome as it creates an unfavorable environment for the growth of pathogenic microbes.44 Studies have shown that vaginal gels containing lactic acid enhance vaginal defense. 45 SilverSol® Vagigel has been formulated with colloidal nanosilver in a gel base containing 0.2% lactic acid to ensure a pH of ~3.5.
Conclusion
In this study, SilverSol® Vagigel was effective and safe in treating the most common vaginal infections i.e. bacterial vaginosis and vulvovaginal candidiasis. Overall, the findings of this study, combined with the existing body of evidence, indicate that SilverSol® Vagigel may represent a valuable addition to the armamentarium of available therapies for vaginal infections, offering potential benefits for patients in terms of improved outcomes and reduced side effects. Overall, SilverSol® Vagigel represents an excellent advancement in women's health, offering a highly effective and safe solution for treating vaginal infections. Leveraging the exceptional antibacterial, antiviral, antifungal, and immune-enhancing properties of SilverSol®, SilverSol® Vagigel formulation can emerge as a promising therapeutic option for treating a wide range of vaginal infections and delivering unparalleled safety and efficacy in addressing diverse feminine health concerns.