Introduction
Intrauterine fetal demise (IUFD) is defined as antenatal or intrapartum fetal death that occurs at or greater than 20 weeks gestation or at a birth weight greater than or equal to 350 grams.1 It is an often unpredictable, distressful event that may have a lasting effect on maternal health. Despite improved antenatal care, IUFD has a 10-fold increased risk of recurrence in future pregnancies.2, 3 However, there is increasing evidence that IUFD, particularly in the third trimester, are largely preventable.1, 4
Currently, less than 5% of stillbirths are recorded globally and yet, it remains the 5th leading cause of death worldwide.1 Developing countries contribute largely to this due to lack of proper access to healthcare providers.5 Despite a fall in the stillbirth rates in the country, India, due to its high population, accounts for the majority of global stillbirths.2, 5
Its incidence varies in literature since gestational age for defining IUFD has historically been non-uniform.1 About 1.9 million cases of IUFD are officially reported worldwide annually.6 However, 76% of these cases are recorded as unexplained IUFDs.1 There is, thus, a limited understanding of the pathophysiology responsible for fetal demise.
Placenta is a readily accessible specimen, which can provide a record of cumulative effects of pregnancy-related events and changes within the intrauterine environment.5, 7 IUFD is multifactorial and as pregnancy progresses, the causes of IUFD are increasingly likely to be extrinsic to the fetus. 4
Placental lesions are important contributors to antepartum IUFD across all gestational ages. There is increasing evidence to show that placental pathology may be the cause of IUFD in pregnancies at or above period of gestation (POG) of 24 week.2 Further, it remains viable for several days after IUFD and can provide information regarding other underlying pathology leading to fetal demise.7 Thus, we aimed to examine placental histopathology in IUFD cases and its role in providing valuable information regarding its causation of IUFD.
Materials and Methods
This was a prospective, descriptive and observational single-center study performed at a tertiary care teaching center in North India for a period of 3 years and 6 months (January 2020 to June 2023). A total of 104 placental specimen of IUFD cases (period of gestation [POG] > 20 weeks) obtained from consenting patients sent for histopathological evaluation to the Department of Pathology were included in this study. Autolysed placental specimen and those with POG of less than 20 weeks were excluded. Patient details including maternal age, POG, presence of pre-existing maternal conditions, presence of any known placental pathology or known fetal abnormalities were recorded from the histopathological case record forms. The Stockholm classification of still-birth was used to identify the possible cause of IUFD in each case based on the available clinical details.8
The placenta specimen was collected immediately after delivery and was transported to the histopathology laboratory in 10% buffered formalin. Placental weight and size of the disc, cords and membranes were recorded. Inspection of fetal surface was done for colour, consistency and translucency of the membranes. Vessels were inspected for the presence of thrombi or calcification. Presence of subchorionic fibrin plaques and deposits were noted. The umbilical cord was examined for the presence of vessels and its point of insertion into the disc was noted. The maternal surface was examined for any significant areas of disrupted cotyledons, fibrosis, infarct or retroplacental hematoma. Serial sectioning of the placental disc from the maternal surface through to the fetal surface looking for infarcts, hemangiomas or other lesions was done. After fixation, standard grossing protocol was followed.9, 10
Histopathological evaluation was performed by 3 independent pathologists on routine hematoxylin and eosin-stained sections using standard definitions established by the Amsterdam Placental Workshop Group.9 The lesions were further classified as maternal vascular malperfusion (MVM) or fetal vascular malperfusion (FVM), inflammatory lesions or idiopathic. The term MVM was used when retroplacental hematoma, decidual vasculopathy, delayed villous maturation and intraparenchymal hematoma were noted. Fetal vascular malperfusion was used in the presence of avascular villi, villous stromal vascular karyorrhexis, occlusive and nonocclusive thrombi in fetal vessels and chorangiomas (fetal vascular lesion). Inflammatory lesions were characterized by the presence of chorioamnionitis (maternal inflammatory response, MIR), fetal inflammatory response (FIR), villitis, intervillositis, and deciduitis. Idiopathic conditions are those in which no other abnormalities could be recognized. Gross findings were confirmed microscopically and all observations were recorded.
The data was tabulated and analyzed using SPSSv23 software. Categorical variables were expressed as percentages. Normally distributed quantitative variables were expressed as mean ± SD and non-normally distributed ones as intervals.
Results
Clinical details
A total of 104 placental specimens from cases with IUFD were evaluated. The average maternal age was 27.2 years. Among these cases the mean gestation age was 28.6 ± 6.3, with 82.7% cases being pre-term. Majority of the cases were seen in multigravida (52.8%).
On the basis of clinical history, ultrasonography findings and other hematological investigations, the causes of IUFD were divided into maternal, fetal, placental and unknown (Table 1, Table 2, Table 3). Of these, maternal causes were the most common (51.9%). Pregnancy induced hypertension (PIH) was the most common maternal cause; congenital anomalies were the most common fetal and placenta previa was the most common placental cause. The rest 20 cases had other causes including post-dated pregnancy and those in which no cause was known.
Placental histopathological findings
Majority cases had a placental weight between 200 and 500 grams (73.1%) with the average placental weight being 277.4 grams. Only 28 cases (26.9%) had weight less than 200 grams and none were more than 500 grams. Majority cases showed eccentric insertion of the cord (71/104, 68.2%).
Table 4 summarizes the microscopic findingsnoted in these cases. Lesions were found in 90.3% of placenta evaluated. MVM was seen in 26 (25%) cases and FVM in 17 cases. A combined MVM and FVM was seen in 3 cases while 10 (9.6%) cases were idiopathic with no specific changes in the placenta. Inflammatory lesions were noted in 48 (46.1%) cases with chorioamnionitis being the most common feature (36/ 104, 34.6%), followed by deciduitis in 15 (14.4%), FIR in 12 (11.5%) and villitis in 5 (4.8%) cases. Features of MVM and inflammatory changes were seen in all gestational ages. Figure 1 demonstrates the common microscopic findings encountered.
Figure 1
Photomicrographs of hematoxylin and eosin-stained sections showing: fibrotic villi (400x, A and 200x, B), calcified villi (400x, C and 200x D), prominent syncytial knots (100x, E), infarct (200x, F), acute chorioamnionitis (100x, G), funisitis (200x, H) and decreased number of villi for age (200x, I)
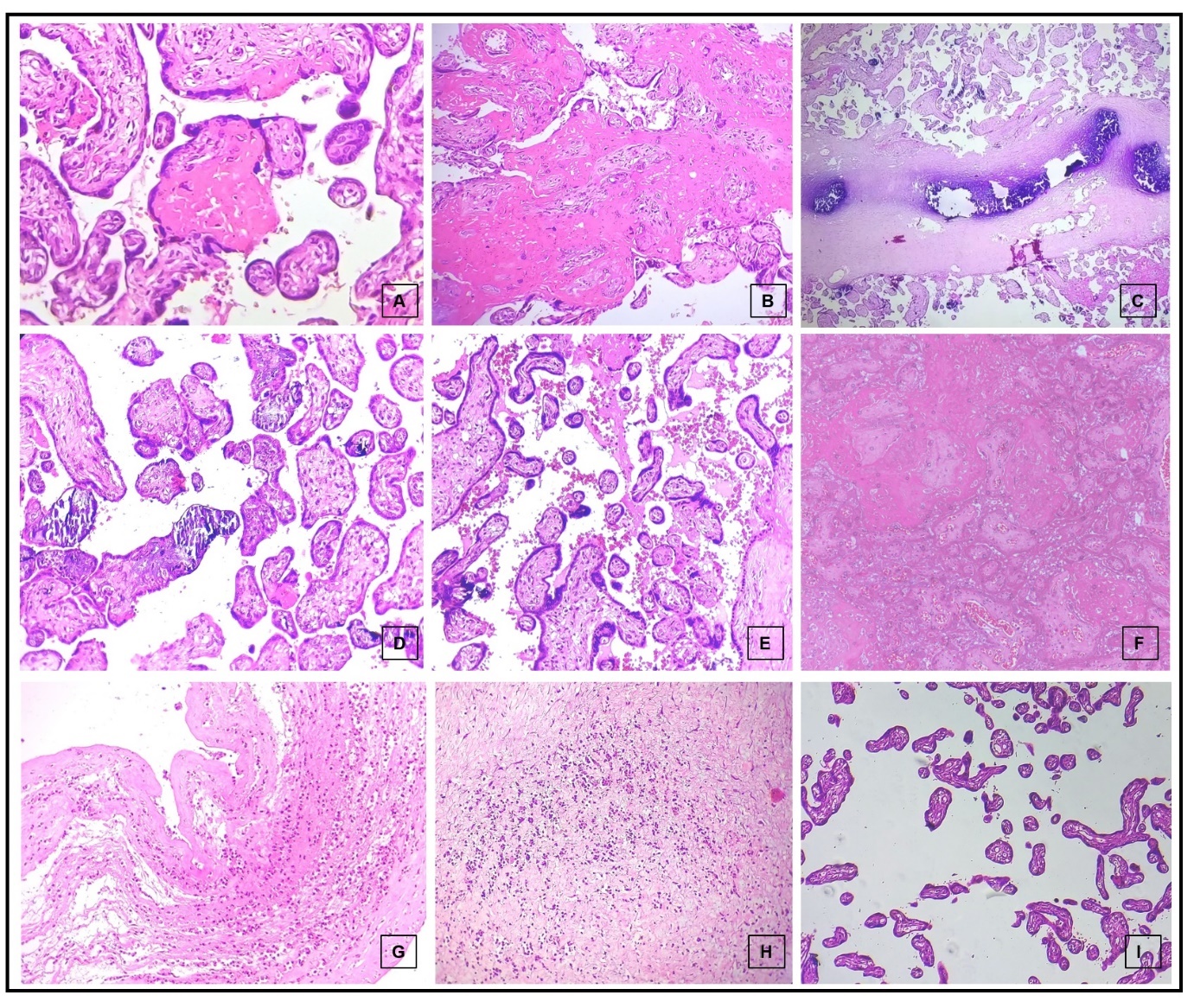
Figure 2
Gross specimen of single case of intra-uterine fetal deaths with diamniotic dichorionic placenta (A). Photomicrographs of hematoxylin and eosin-stained sections showing meconium macrophages (1000x, B), atypical cells of acute promyelocytic leukemia (400x, C) and umbilical cord with two blood vessels (40x, D)
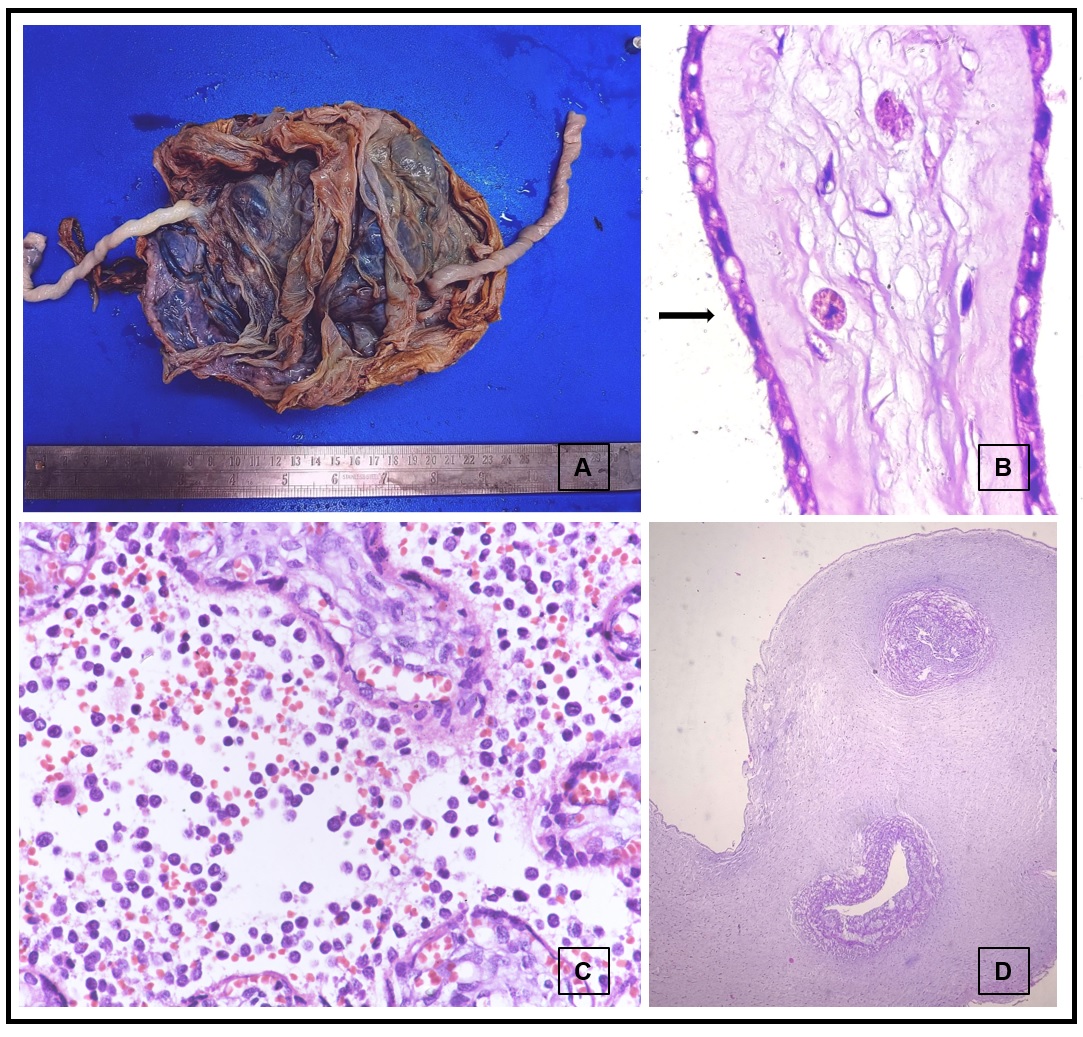
Table 1
Maternal causes of intra-uterine fetal death
Table 2
Fetal causes of intra-uterine fetal death
Table 3
Placental causes of intra-uterinefetal death
|
Placental Causes |
N (%) |
|
Placenta Previa |
4 (33.3) |
|
Abruptio Placenta |
3 (25) |
|
Premature Rupture of Membranes |
2 (16.7) |
|
Oligohydroamnios |
2 (16.7) |
|
Placenta Percreta |
1 (8.3) |
|
Total |
12 (100) |
Table 4
Histopathological characteristics of placenta in cases of intra-uterine fetal death
Discussion
IUFD occurs as a result of a complex process involving the mother, the fetus as well as the placenta.7 Hence, a comprehensive analysis of all these factors is necessary to determine the cause of fetal demise. In our study, we evaluated all 3 factors in 104 cases of IUFD.
Increased risk of IUFD is seen in advanced maternal age, presence of chronic maternal illness, poor socioeconomic status and multiple gestations.1 We found IUFD to be higher in women between 25-30 years of age. Similar findings were reported by Manocha et al. However, they reported a higher incidence of IUFD in primigravida, while we found that majority of our cases were multigravida.2 There is wide variation in reported literature from India regarding the same.7, 11 However, our findings are similar to those reported by Kanavi et al and other international researchers.12 The mean gestation age was 28.6 weeks in our study, with majority being pre-term (82.7%), which was in concordance with the findings of Borade et al and Manocha et al.2, 7
PIH was the most common maternal cause of IUFD (20.3%) in our study. Congenital anomalies associated with IUFD were the most common underlying cause amongst the fetal causes (72.1%) and was also the most common single cause overall. Placenta previa was the most common placental cause (33.3%). Borade et al found PIH to be the most common maternal cause of death. Abruptio placenta was the most common placental cause identified by them, while no specific fetal cause was identified.7 However, our findings were similar to those of Singh et al and Khashoggi et al.13, 14 Singh et al also identified congenital anomalies as the most common fetal cause of IUFD and PIH as the most common maternal cause.13
Following IUFD, regardless of etiology, the placenta undergoes certain post-mortem changes due to cessation of fetal blood flow, alteration of maternal perfusion, maternal inflammatory response to non-viable products and labor changes associated with separation of placenta. Cessation of blood flow leads to endothelial karyorrhexis, loss of luminal integrity, and extravasation of erythrocyte fragments onto the villous stroma, which over time ultimately results in avascular villi. Muscular fetal vessels in stem villi and the chorionic plate also undergo obliteration. Initially, following IUFD, maternal perfusion persists but over time, it diminishes and results in ischemic changes including villous fibrosis with diminished cellularity, and replacement of the maternal intervillous space by fibrin. In cases of IUFD prior to the initiation of labor, there may be changes secondary to placental separation during the third stage of labor such as presence of adherent retroplacental blood and recent placental infarction noted microscopically.15
Placental pathology in maternal causes
PIH
Presence of PIH was seen in 20.3% cases, while 2 patients had chronic hypertension. Pre-eclampsia has also been reported as a leading cause of IUFD by Borade et al and Prasanna et al.7, 16 Majority cases with pre-eclampsia were pre-term (75%). We also found that on an average, placentae were smaller in cases with PIH. On gross examination, they showed presence of calcification, retroplacental hematoma and multifocal infarcts. Microscopic evaluation revealed presence of prominent syncytial knots, calcification, necrosis and infarcts. These findings were in concordance to those reported by previous studies. 7, 16, 17
Maternal COVID-19 (SARS-CoV-2 Virus) infection
In patients with COVID-19 infection (9.3%), it was observed that the placenta showed atleast one of the features of MVM. Grossly, retroplacental hematoma and multiple tan yellow areas were identified. Retroplacental hematoma, intraparenchymal hemorrhage, villous edema, intervillous fibrin, chorioamnionitis, presence of fibrotic villi with extensive perivillous fibrin and necrosis was noted. These findings are in concordance with the studies conducted on the placental tissues of patient of COVID-19 by Shanes et al. and Joshi et al. 18, 19
Bad obstetric history (BOH)
A total of 12.9% patients had significant BOH. This was higher than the numbers reported in previous studies. (20,21) This could be due to the older sample population in our study. All of these cases were in early pre-term POG and showed presence of fibrotic villi with 4 cases showed decreased number of villi.
Anemia
Only 12.9% cases showed anemia, which was lower than those reported by the previous studies and could be related to the better socio-economic status of our patient population. Microscopic evaluation revealed infarction and calcification similar to previous studies.7, 20, 21 However, we did not observe any retroplacental hematoma in these cases.
Sepsis
On evaluation of the cases with sepsis (5.6%), tan-white discoloration was noted in the membranes and fetal surface. Microscopically, presence of bacterial colonies in the villi associated with necrosis and villitis, bacterial colonies and necrosis within the umbilical cord and necrotizing chorioamnionitis were noted.
Other maternal causes
The placenta in IUFD cases with maternal hypothyroidism showed were less than 200 grams in weight. This is in concordance to the findings of Kumari et al.22 They further displayed presence of decreased number of villi and hypovascular villi, which has also been described in previous studies.23
In cases of Rh incompatibility, presence of hydropic villi and intervillous hemorrhage was noted, which was similar to the findings of Borade et al.7 The frequency of cases in our study was similar to that found in other studies in the Indian population.24
Maternal gestational diabetes mellitus (GDM) was noted in 2 cases only. Both of these showed calcification and presence of only 2 blood vessels grossly, which was confirmed microscopically (Figure 2). They also showed features of chorioamnionitis of membrane, fibrotic villi and intervillous hemorrhage. These findings are similar to those noted in other studies.16, 21
In cases of maternal intrahepatic cholestasis of pregnancy (IHCP) (3.7%), presence of perivillous fibrin deposition and chorioamnionitis was noted. One case also showed presence of chronic deciduitis and the other showed presence of prominent syncytial knots. The incidence of IHCP in our study and the microscopic findings are in concordance with those previously described in literature.25, 26
Atypical cells were noted in the intervillous and intravillous space of the single case of maternal acute promyelocytic leukemia (Figure 2).
Placental pathology in placental and fetal causes
Abruption
Retroplacental hematoma and infarcts were noted on gross evaluation of all three cases of abruption while calcification was noted in only one case. Microscopically, fibrotic villi, perivillous fibrin deposition, intervillous hemorrhage, stromal fibrosis, fibrin thrombi and features of infarction were noted. Two cases also showed chorionitis. Avascular villi with presence of calcification were noted in 1 case. These findings were in accordance to the previous studies.7, 16
Intra-uterine growth retardation (IUGR)
Grossly, the 3 cases IUGR showed focal calcification and multiple grey white areas of infarction were identified in 2 cases. Microscopically, presence of distal villous hypoplasia was noted. Also noted were features of infarction and increased vascularity indicating presence of prolonged fetal hypoxia.
Other placental lesions
In patients with placenta previa, fibrotic villi, focal calcification, hypovascular villi and presence of chorioamnionitis were major findings. While in oligohydroamnios, fibrotic villi with focal calcification, chorioamnionitis, deciduitis and vascular luminal narrowing was noted. In premature rupture of membranes (PROM), predominantly inflammation of the membranes was seen.
Other fetal causes
Both cases with meconium-stained liquor (MSL) showed presence of meconium macrophages in the membranes (Figure 2) suggesting long standing fetal hypoxia. They also showed presence of calcification in the membranes along with acute chorioamnionitis.
In our study, inflammatory lesions were found to be most common followed by MVM. Further, no particular pattern was noted in the presence of these features and the gestational age of the fetus. This is in contrast to the findings of Manocha et al, who found MVM to be the most common finding in placental tissue associated with IUFD and they further found that MVM was found to be more common in early preterm cases.2 This discrepancy could be explained by the difference in the demographic characteristic of their sample population and ours.
Inflammatory lesions of the placenta may be due to infectious etiology (viral, bacterial and parasitic) as well as immune origin, such as maternal anti-fetal rejection.27 These conditions, when detected in time may be prevented by early initiation of management of the immune response leading to IUFD.
The major limitation of our study was the lack of clinical details in several patients due to the incomplete filling of the histopathological requisition form. Further, due to the outbreak of the COVID-19 pandemic during the study and with the conversion of our center into a COVID-19 facility, obtaining these details became more difficult and also severely limited our sample size.
Conclusion
A comprehensive placental histopathological evaluation can provide clues regarding the causation and progression of IUFD. In our study, maternal causes were found to be the most common and fetal congenital anomalies were major contributors to IUFD. PIH was found to be the major maternal cause, while placenta previa was the most common placental abnormality seen. These pathologies are associated with histopathological placental lesions often leading to vascular insufficiency and abnormal fetal development. Predominance of placental inflammatory lesions in our study points towards presence of modifiable causes that can be addressed directly. An evaluation of placental features can not only identify the cause of IUFD but also, possibly help prevent recurrence in following pregnancies by allowing timely initiation of screening and management. The identification of a probable cause and patient education regarding the same may also motivate the patient to seek early and proper antenatal care in subsequent pregnancies.


