- Visibility 63 Views
- Downloads 11 Downloads
- DOI 10.18231/j.ijogr.2025.009
-
CrossMark
- Citation
Changes in doppler parameters in severe fetal growth restriction and its association with perinatal outcomes in an Indian tertiary care centre
Introduction
Fetal growth restriction (FGR) refers to a condition in which a fetus is not able to acquire its genetically determined growth potential.[1] FGR is the most common and complex problem in present-day obstetrics.[2] The prevalence of FGR infants is said to be as high as 26% in India.[3]
A fetus is labeled as small for gestation (SGA) when estimated fetal weight and abdominal circumference (AC) is <10th centile for the gestational age.[4]
SGA fetus that has AC between 3rd & 10th centile is most likely to be a small and healthy fetus and have near-normal perinatal outcome whereas fetus with AC less than 3rd centile is truly or severely growth restricted.[4] Seventy percent of the SGA fetuses are constitutionally small (healthy SGA) or have a milder form of fetal growth restriction (FGR) with normal Doppler parameters. The causes of FGR can be divided into maternal, uteroplacental, and fetal conditions. Utero-placental group (FGR due to placental insufficiency) appears to be the largest group and is mainly a vascular disorder.[5] It begins with abnormal tertiary villous vessels and terminates with characteristic fetal multiple vascular and cardiac manifestations.[6] These manifestations can be recorded with Doppler examination of the following vessels:
Fetal - Umbilical artery, Middle cerebral artery, Ductus venosus, and Umbilical vein.
Maternal - Uterine artery.
Fetal Doppler studies have been well studied, however, few studies have looked at the serial changes in the Doppler parameters in pregnancies with severe FGR which are caused by placental insufficiency. The study of serial changes in doppler parameters in pregnancies with severe FGR gives insight regarding the pattern of changes in doppler parameters and helps in the management of these pregnancies. To the best of our knowledge, there are only a few studies on the subject and there is a paucity of Indian data.
Materials and Methods
A prospective observational study was carried out in the Department of Obstetrics and Gynaecology at a tertiary care hospital between years 2020-2021. A sample size of 50 was initially planned but due to COVID-19 pandemic in 2020-2021, a study population of 40, included patients attending Antenatal clinic and admitted in wards was taken.
The study was conducted on pregnant women attending antenatal clinics and admitted in maternity wards and labour room at Lok Nayak hospital with an aim to study serial changes in the Doppler parameters in pregnancies with severe fetal growth restriction from the diagnosis till the termination of pregnancy in the Umbilical Artery, Middle Cerebral Arteries, Ductus venosus & Umbilical vein and Uterine artery. A total of 52 women with pregnancy at 28 to 34 weeks of gestation who had foetal growth lagging behind by 4 weeks on clinical examination were assessed for eligibility. Of 52 women, 12 women did not meet eligibility criteria and 40 women who met all eligibility criteria with EFW or AC < 3rd centile were enrolled in the study.
After checking the fulfilment of inclusion criteria, eligible subjects were assessed by serial Doppler studies. The Doppler was performed by trans-abdominal method using trans-abdominal probe of 2-5 MHz.
The Doppler assessment used a 2-5 MHz trans-abdominal probe to examine vessels, employing color Doppler to identify them and obtain spectral traces. Recordings were taken during fetal immobility without breathing, averaging three consecutive Doppler velocity waveforms per vessel. Umbilical artery and vein, Middle Cerebral Artery near the circle of Willis, and Uterine Artery were sampled.
Pulsatility Index (PI) were determined in the UA, bilateral UtA and the fetal MCA at each visits. For analysis purpose, the total study population was divided into groups according to gestational age such as 28-29+6 weeks, 30-31+6 weeks, 32-33+6 weeks, 34-35+6 weeks and 36-37+6 weeks and mean, median and range of PI in these vessels were calculated. Patients were tracked until delivery, and perinatal outcomes were assessed. Pregnancy termination decisions adhered to institutional protocols:
The primary outcome was to identify proportion of patients with pulsatility index >95th centile in Umbilical and Uterine artery, proportion of those with < 5th centile in Middle Cerebral artery, patients with absent or reversal of a wave in ductus venosus and presence of pulsation in Umbilical vein. While the secondary outcome was to assess mean period of gestation at the onset of Doppler abnormality, the percentage of pregnancies completing at least 37 weeks gestation and adverse perinatal outcomes such as the percentage of cesarean sections in view of fetal distress, fresh stillbirths attributed to birth asphyxia, APGAR score at 1 and 5 minutes, neonates requiring NICU admission and duration of stay.
Sample size
Calculated for abnormality in Doppler parameters considering the rate of abnormal Doppler to be in 30% of cases and power of 80%, a sample size of 86 was calculated.
Formula used:
n=Z2 pq /d2
1.96*1.96*0.3*0.7/0.04*0.04 = 86
Due to constrain of time a convenient sample size of 50 subjects was planned. However, because of the COVID-19 pandemic, we could enroll 40 subjects in our study.
Data collection and management
Data were collected on a predesigned proforma (annexure 1) and were entered in the excel sheet.
Statistical methods
The collected data was entered in Microsoft Excel and then analyzed and statistically evaluated using the SPSS-25 version. The normality of each variable was assessed by using the Kolmogorov- Smirnov test and the Shapiro-Wilk test. Quantitative data were expressed by mean, standard deviation or median with interquartile range. Qualitative data was expressed in percentages and the difference between the proportions was tested by the chi-square test or Fisher’s exact test. ‘P’ value less than 0.05 was considered statistically significant. Variance of interval in doppler abnormalities will be analysed by ANOVA.
Ethical considerations
Approval from the institutional research ethics committee was taken prior to starting the trial. CTRI registration number for the trial: CTRI/2020/07/026377.
Results
A total of 40 pregnancies complicated with FGR full-filled the inclusion criteria of the study. [Table 1] depicts the demographic characteristics of the study population. The mean age of the total study population was 27 ± 4.49 years. Most of the subjects had completed secondary education (62.5%), belonged to upper lower (47.5%) and lower middle (50%) socioeconomic status by modified Kuppuswamy scale and were housewife by occupation (80%).
|
Parameter |
Value |
|
Maternal age (years, mean (range)) |
27 ± 4.49 (19-37) |
|
Literacy (n (%)) |
|
|
Illiterate |
1 (2.5) |
|
Primary |
7 (17.5) |
|
Secondary |
25 (62.5) |
|
Higher secondary |
7 (17.5) |
|
Socioeconomic status (n (%)) |
|
|
Upper lower |
19 (47.5) |
|
Lower middle |
20 (50) |
|
Upper middle |
1 (2.5) |
|
Occupation (n (%)) |
|
|
Housewife |
32 (80) |
|
Others |
8 (20) |
Obstetric and clinical details
Out of 40 patients in the study, 57.5% (n=23) were primigravida, 25% (n=10) were second gravidas, 12.5% (n=5) were third gravidas, and 5% (n=2) were fourth gravidas. The mean weight and height of the subjects were 53.58 ± 5.73 kg and 155.83 ± 4.78 cm, with a mean BMI of 21.84 ± 1.61 kg/m2. BMI was below 20 kg/m2 in 12.5% of subjects, with only one patient having a BMI above 25 kg/m2. The mean gestational age at recruitment was 31 weeks and 1 day, ranging from 28 to 34 weeks, with 67.5% (n=27) enrolled cases being less than 32 weeks gestation. The mean gestational age at delivery was 33 weeks and 2 days, ranging from 29 to 37 weeks. ([Table 2])
|
Parameter |
Value |
|
Parity (n (%)) |
|
|
0 |
23 (57.5) |
|
1 |
10 (25) |
|
2 |
5 (12.5) |
|
3 |
2 (5) |
|
Weight (kgs, mean (range)) |
53.58 ± 5.73 (43-65) |
|
Height (cms, mean (range)) |
155.8 ± 4.78 (149-168) |
|
BMI (kg/m2, mean (range)) |
21.84 ± 1.61 (18.5-25.6) |
|
Gestational age at the time of recruitment (weeks + days) |
31+1 (28-34) |
|
Gestational age at the time of delivery (weeks + days) |
33+2 (29-37) |
Out of 17 multigravida cases, 17.64% women (n = 3) had history of FGR in previous baby while rest 82.35% (n = 14) had new onset FGR in current pregnancy. 45% of the study subjects (n = 18) had associated hypertensive disorders of pregnancy. Out of these 18 subjects, 9 subjects had gestation hypertension and rest 9 had preeclampsia. 77.78% of the subjects (n = 14) had period of gestation less than 32 weeks and rest 22.22% subjects (n = 4) had POG more than 32 weeks.
Doppler examination details of the study population
Umbilical artery pulsatility index (UA PI)
As gestation progressed, there was a gradual rise in resistance to blood flow in the umbilical artery. Serial examinations revealed that abnormalities detected earlier in gestation progressed more rapidly, while those emerging later progressed slower as shown in [Table 3]. Additionally, in late-onset FGR fetuses, UA PI can remain normal.
|
POG (weeks) at the time of first examination |
Total no. of cases (n) |
No. & percentage having UA PI > 95 th centile n (%) |
Mean ± SD of UA PI (>95th centile) |
Median & range of UA PI (>95th centile) |
||
|
Positive EDV |
AEDF |
REDF |
||||
|
28-29+6 |
15 |
8 (53.33) |
3 (20) |
0 (0) |
1.83 ± 0.55 |
1.56 (1.47- 2.58) |
|
30-31+6 |
12 |
7 (58.3) |
0 (0) |
0 (0) |
1.57 ± 0.15 |
1.46 (1.36-1.59) |
|
32-33+6 |
12 |
2 (16.7) |
0 (0) |
0 (0) |
1.54 ± 0.09 |
1.54 (1.47-1.60) |
|
34-35+6 |
1 |
0 (0) |
0 (0) |
0 (0) |
- |
- |
|
Total |
40 |
20 (50) |
3 (7.5) |
0 (0) |
- |
- |
|
Second examination |
||||||
|
28-29+6 |
5 |
0 (0) |
2 (40) |
3 (60) |
2.91 ± 0.99 |
2.60 (2.-3.80) |
|
30-31+6 |
15 |
9 (60) |
6 (40) |
0 (0) |
2.08 ± 0.72 |
1.65 (1.47-2.56) |
|
32-33+6 |
9 |
9 (100) |
0 (0) |
0 (0) |
1.56 ± 0.13 |
1.56 (1.50-1.62) |
|
34-35+6 |
11 |
4 (36.4) |
0 (0) |
0 (0) |
1.55 ± 0.12 |
1.55 (1.45-1.65) |
|
Total |
40 |
22 (55) |
8 (20) |
3 (7.5) |
|
- |
|
Third examination |
||||||
|
28-29+6 |
2 |
0 (0) |
1 (50) |
1 (50) |
3.04 ± 1.04 |
3.05 (2.31-3.78) |
|
30-31+6 |
13 |
1 (7.6) |
6 (46.2) |
6 (46.2) |
2.89 ± 0.55 |
2.65 (2.46-3.20) |
|
32-33+6 |
13 |
2 (15.4) |
11 (84.6) |
0 (0) |
2.22 ± 0.38 |
2.36 (2.22-2.41) |
|
34-35+6 |
6 |
2 (33.33) |
2 (33.33) |
0 (0) |
1.88 ± 0.46 |
1.89 (1.48-2.28) |
|
36-37+6 |
5 |
1 (20) |
0 |
0 (0) |
1.71 |
1.71 |
|
Total |
39 |
6 (15.38) |
20 (51.28) |
7 (17.95) |
- |
- |
|
Fourth examination |
||||||
|
30-31+6 |
7 |
0 (0) |
0 (0) |
7 (100) |
3.53 ± 0.16 |
3.47 (3.42-3.61) |
|
32-33+6 |
11 |
0 (0) |
3 (27.3) |
8 (72.7) |
3.24 ± 0.54 |
3.26 (2.80-3.76) |
|
34-35+6 |
1 |
0 (0) |
1 (100) |
0 (0) |
2.51 |
2.51 |
|
36-37+6 |
9 |
3 (33.33) |
0 (0) |
0 (0) |
1.66 ± 0.12 |
1.61 (1.57-1.79) |
|
Total |
28 |
3 (10.71) |
4 (14.29) |
15 (53.57) |
- |
- |
Uterine artery PI
In our study, 17 subjects had abnormal uterine artery PI at enrolment, with gestational ages ranging from 28 to 32 weeks. No subjects enrolled beyond 32 weeks exhibited UtA PI > 95th centile. As gestation progressed, subjects with abnormal UtA PI continued to show this derangement. However, no new cases of UtA PI derangement developed in subsequent examinations ([Table 4]).
|
POG (weeks) at the time of first examination |
Total no. of cases |
Left UtA PI > 95th centile n (%) |
Right UtA PI > 95th centile n (%) |
Mean ± SD of UtA PI (>95th centile) |
Median & range of UtA PI (>95th centile) |
|
28-29+6 |
15 |
9 (60) |
9 (60) |
1.31± 0.08 |
1.44 (1.24-1.39) |
|
30-31+6 |
12 |
2 (16.7) |
4 (33.3) |
1.23 ± 0.12 |
1.23 (1.12-1.31) |
|
32-33+6 |
12 |
2 (16.7) |
2 (16.7) |
1.25 ± 0.09 |
1.26 (1.18-1.35) |
|
34-35+6 |
1 |
0 |
0 |
- |
- |
|
Total |
40 |
13 |
15 |
|
|
|
Second examination |
|||||
|
28-29+6 |
5 |
3 (60) |
4 (80) |
1.37 ± 0.08 |
1.38 (1.21-1.48) |
|
30-31+6 |
15 |
7 (46.7) |
7 (46.7) |
1.26 ± 0.07 |
1.28 (1.21-1.37) |
|
32-33+6 |
9 |
3 (33.3) |
4 (44.1) |
1.29 ± 0.04 |
1.29 (1.25-1.34) |
|
34-35+6 |
11 |
0 |
0 |
- |
- |
|
Total |
40 |
13 |
15 |
|
|
|
Third examination |
|||||
|
28-29+6 |
2 |
2 (100) |
2 (100) |
1.29 ± 0.13 |
1.29 (1.18-1.52) |
|
30-31+6 |
13 |
6 (46.2) |
7 (53.8) |
1.25 ± 0.07 |
1.24 (1.18-1.36 |
|
32-33+6 |
13 |
4 (30.8) |
5 (38.5) |
1.23 ± 0.06 |
1.24 (1.13-1.27) |
|
34-35+6 |
6 |
0 |
0 |
- |
- |
|
36-37+6 |
5 |
0 |
0 |
- |
- |
|
Total |
39 |
12 |
14 |
|
|
|
Fourth examination |
|||||
|
30-31+6 |
7 |
3 (42.9) |
4 (57.1) |
1.24 ± 0.08 |
1.24 (1.13-1.35) |
|
32-33+6 |
11 |
3 (27.3) |
4 (36.4) |
1.12 ± 0.21 |
1.24 (0.61-1.26) |
|
34-35+6 |
1 |
0 |
0 |
- |
- |
|
36-37+6 |
9 |
0 |
0 |
- |
- |
Middle cerebral artery
In our study, 4 subjects (26.66%) had MCA PI < 5th centile (brain sparing) at enrolment, with gestational ages ranging from 28 to 28+6 weeks ([Table 5]). No subjects in other groups exhibited abnormal MCA PI at enrolment. However, as gestation progressed, the number of subjects with abnormal MCA PI increased progressively in subsequent examinations. By the final examination, brain sparing was observed in all 40 subjects, regardless of their gestational age at enrolment. This study revealed a progressive reduction in resistance to blood flow in the middle cerebral artery (brain sparing) in both early and late-onset FGR fetuses.
|
POG (weeks) at the time of first examination |
Total no. of cases |
MCA PI < 5th centile n (%) |
Mean ± SD of MCA PI (<5th centile) |
Median & range of MCA PI (< 5th centile) |
|
28-29+6 |
15 |
4 (26.66) |
1.27 ± 0.12 |
1.24 (1.20-1.35) |
|
30-31+6 |
12 |
0 (0) |
- |
- |
|
32-33+ 6 |
12 |
0 (0) |
- |
|
|
34-35+6 |
1 |
0 (0) |
- |
- |
|
Total |
40 |
4 (10) |
|
|
|
Second examination |
||||
|
28-29+6 |
5 |
5 (100) |
1.38 ± 0.14 |
1.41 (1.27-1.50) |
|
30-31+6 |
15 |
8 (53.3) |
1.40 ± 0.09 |
1.41 (1.34-1.46) |
|
32-33+6 |
9 |
6 (66.7) |
1.36 ± 0.11 |
1.35 (1.28-1.45) |
|
34-35+6 |
11 |
8 (72.7) |
1.16 ± 0.20 |
1.11 (1.00-1.32) |
|
Total |
40 |
27 (67.5) |
|
|
|
Third examination |
||||
|
28-29+6 |
2 |
2 (100) |
1.35 ± 0.16 |
1.35 (1.24-1.46) |
|
30-31+6 |
13 |
13 (100) |
1.34 ± 0.10 |
1.38 (1.31-1.41) |
|
32-33+6 |
13 |
13 (100) |
1.31 ± 0.09 |
1.31 (1.26-1.35) |
|
34-35+6 |
6 |
5 (83.3) |
1.17 ± 0.14 |
1.23 (1.05-1.29) |
|
36-37+6 |
5 |
5 (100) |
1.04 ± 0.15 |
0.99 (0.96-1.06) |
|
Total |
39 |
38 (97.44) |
|
|
|
Fourth examination |
||||
|
30-31+6 |
7 |
7 (100) |
1.21± 0.09 |
1.18 (1.15-1.32) |
|
32-33+6 |
11 |
11 (100) |
1.26 ± 0.07 |
1.26 (1.21-1.30) |
|
34-35+6 |
1 |
1 (100) |
1.24 |
1.24 |
|
36-37+6 |
9 |
9 (100) |
1.0 ± 0.14 |
0.94 (0.91-1.12) |
|
Total |
28 |
28 (100) |
|
|
Ductus venosus doppler and umbilical vein pulsations
Ductus venosus and umbilical vein doppler was normal in all subjects at the time of first examination. In subsequent examinations, 4 subjects had absent ‘a’ wave in ductus venosus and all of them belonged to 28-29+6 and 30-30+6 period of gestation group. In one subject, absent a wave deteriorated to reversal within one day. In all subjects, pregnancy was terminated as soon as absent or reversal of ‘a’ wave in DV or REDF was observed.
It was also observed that by the time of delivery, most of the subjects (n = 34, 85%) had elevated UA PI, all patients had reduced MCA PI (n = 40, 100%), 7.5% had absent a wave in DV and 2.5% had reversal of a wave in DV. None of them had visible pulsations in umbilical vein. Longitudinal cumulative onset time curves of abnormal doppler parameters calculated for each vessel is shown in [Figure 1].
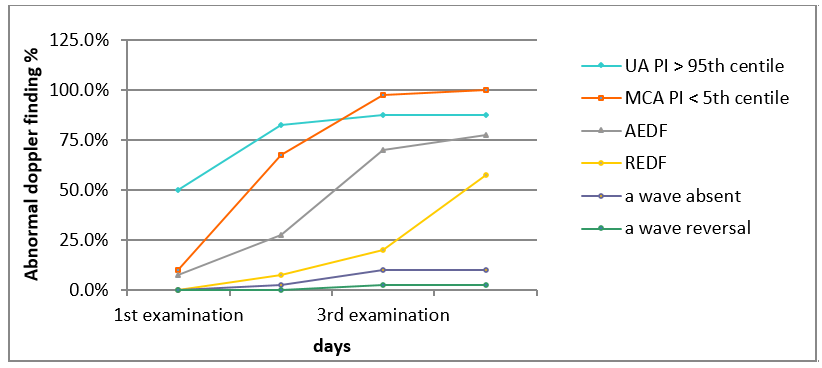
Progression pattern for the doppler abnormalities in the study population
Three principal patterns of deterioration in Doppler parameters were observed based on the degree of placental insufficiency. Firstly, mild placental insufficiency was identified in 15% (n=6) of subjects, all presenting with isolated abnormal MCA PI (brain sparing), enrolled at a median gestational age of 33.5 weeks and delivering at a median of 37 weeks. Secondly, progressive placental insufficiency, observed in 70% (n=28) of subjects, showed mild onset but progressive abnormality in cerebral and umbilical circulation. These subjects were enrolled at a median gestational age of 30.4 weeks and delivered at a median of 33.2 weeks. Finally, severe early-onset placental insufficiency, observed in 15% (n=6) of subjects, exhibited severe cardiovascular compromise early in gestation and rapid progression. These subjects were enrolled at a median gestational age of 29 weeks.
The median interval from the occurrence of an elevated UA PI (>95th centile) with positive EDV, elevated UtA PI (>95th centile), reduced MCA PI (<5th centile) and AEDF in UA were 12, 15, 6 and 3 days, respectively, with a statistically significant difference overall (ANOVA P value <0.0001).
Adverse perinatal outcomes
In our study, a significant correlation was found between the degree of abnormality in umbilical artery doppler and adverse perinatal outcomes (p-value <0.0001). Out of 40 subjects, two subjects had intrauterine demise and REDF was present in both of them. Out of the remaining 38, 73% had elective LSCS, 81% had preterm delivery and 57% had NICU admission for more than 48 hours. Two early neonatal death were seen and both of them had abnormalities in DV doppler. There was no significant correlation between UtA doppler and adverse perinatal outcomes. MCA PI was abnormal in all subjects at the time of delivery. So, we could not compare perinatal outcomes with regard to MCA doppler.
When perinatal outcomes for early vs late onset FGR were compared, more no. of early onset FGR fetuses were delivered preterm, had poor 1 minute Apgar and more prolonged hospital stay.
Discussion
Our study on singleton pregnancies with fetal growth restriction (FGR) below the 3rd centile for estimated fetal weight or abdominal circumference reveals a consistent longitudinal pattern of doppler parameter abnormalities in maternal and fetal vessels, including UtA, UA, MCA, and DV. These abnormalities progress gradually prior to delivery, offering potential predictive value for adverse perinatal outcomes. While existing cross-sectional studies link abnormal doppler parameters with adverse outcomes, our longitudinal approach allows for early detection of severe cardiovascular changes in FGR fetuses, filling a gap in understanding progression patterns. Notably, our findings align with demographic and clinical variables reported in existing literature, enhancing their applicability.
In our study, as gestational age at enrolment advanced, fewer subjects exhibited deranged umbilical artery PI > 95th centile. FGR detected before 32 weeks showed more severe placental insufficiency, with a significant relationship between the rate of progression for doppler abnormalities and gestational age. Early-onset abnormalities progressed more rapidly to AEDF and REDF, while later-onset abnormalities showed slower progression, consistent with findings by Turan et al.[7] Brain sparing, evidenced by progressive reduction in MCA resistance, was observed in all subjects regardless of gestational age at enrolment, contrary to Rosello et al's study.[8] Fifteen percent of subjects had normal UA PI but isolated abnormal MCA PI, resembling findings by Turan et al, likely indicating mild placental insufficiency not reflected in UA PI. In our study, uterine artery derangement was significantly associated with hypertensive disorders of pregnancy, persisting as gestation advanced, with no new onset derangements noted as was observed by Maroni et al.[9] These findings underscore the pathogenesis of FGR in hypertensive disorders and highlight the utility of uterine artery examination, a component often overlooked in previous studies.
In our study, 85% of subjects followed the classical sequence of abnormal doppler parameters, starting with increased arterial resistance, followed by brain sparing, and sometimes followed by venous deterioration. Only 15% showed a non-classical sequence, with abnormal MCA PI being the sole abnormality. This differs from findings by Baschat et al (2001)[10] and Rosello et al (2017), which reported variations in the sequence and observed UV pulsations. However, Unterscheider et al (2013) questioned the predictability of doppler deterioration sequences, noting no significant difference in the frequency of classical versus other sequences, though their study differed in sample characteristics and inclusion criteria.[11]
In our study, the progression rate of doppler deterioration in FGR varied with gestational age and placental insufficiency severity. Beyond 32 weeks, subjects mainly showed umbilical and MCA abnormalities, leading to termination near term. Before 30 weeks, rapid arterial and venous abnormalities required early termination, while around 30 weeks, mild but progressive abnormalities led to termination between 32-35 weeks. Median intervals from abnormal doppler parameters to delivery differed from Turan et al and Rosello et al, possibly due to sample variations. Cumulative onset time curves were consistent with Ferrazzi et al's findings.[12]
In our study, a significant correlation (p < 0.0001) was observed between abnormal umbilical artery doppler and adverse perinatal outcomes, with two intrauterine demises associated with REDF and two early neonatal deaths linked to DV abnormalities as seen by Westergaard et al.[13] Late doppler changes like REDF and absent/reversal of a wave in DV emerged as the best predictors of perinatal outcomes, consistent with findings by Ferrarzzi et al and Unterscheider et al. However, MCA PI abnormalities were universal at delivery, precluding outcome comparison. No significant correlation was found between UtA doppler and adverse perinatal outcomes which were not observed in study conducted by Rai L[14] and Shwarzman P et al.[15]
Mostly studies have looked at the cross sectional data and there are only few studies which have looked at the serial changes in doppler parameters in pregnancies with severe FGR and to the best of our knowledge, there is no study in an Indian setting.
Our study highlights two key conclusions regarding fetal growth restriction (FGR): firstly, the progression rate of doppler deterioration is influenced by both gestational age and the severity of placental insufficiency at the time of onset. Secondly, in FGR subjects with AC or EFW below the 3rd centile, abnormalities in doppler parameters exhibit a consistent and longitudinally related pattern in maternal and fetal vessels. These findings underscore the importance of early detection and monitoring of doppler parameters in FGR management.
Conclusion
The following conclusions are drawn from our study:
The progression rate of doppler deterioration in FGR is determined by the period of gestation and degree of placental insufficiency at the time of onset.
In FGR subjects with AC or EFW less than 3rd centile, abnormalities in doppler parameters occur in a longitudinally related and consistent pattern in maternal and fetal vessels.
Source of Funding
None.
Conflict of Interest
None.
References
- E Ferrazzi, T Stampalija, K Makarenko, D Casati. Fetal Growth Restriction (FGR)-Fetal Evaluation and Antepartum Intervention. Curr Obstet Gynecol Rep 2013. [Google Scholar]
- . ACOG Practice Bulletin No. 204: Fetal Growth Restriction. Obstet Gynecol 2019. [Google Scholar]
- VG Kanabar, MS Patel, SR Shah, SK Jani. Analytical study of 50 cases of fetal growth restriction. Int J Adv Med 2014. [Google Scholar]
- F Figueras, E Gratacos. An integrated approach to fetal growth restriction. Best Pract Res Clin Obstet Gynaecol 2017. [Google Scholar]
- M Alberry, P Soothill. Management of fetal growth restriction. Arch Dis Child Fetal Neonatal Ed 2007. [Google Scholar]
- DA Baschat. Fetal responses to placental insufficiency: an update. BJOG 2004. [Google Scholar]
- O Turan, S Turan, S Gungor, C Berg, D Moyano, U Gembruch. Progression of Doppler abnormalities in intrauterine growth restriction. Ultrasound Obstet Gynecol 2008. [Google Scholar]
- J Morales-Roselló, A Khalil, V Fornés-Ferrer, J Alberola-Rubio, D Hervas-Marín, NP Llorens. Progression of Doppler changes in early-onset small for gestational age fetuses. How frequent are the different progression sequences. J Matern Fetal Neonatal Med 2018. [Google Scholar]
- E Maroni, A Youssef, T Arcangeli, M Nanni, E Contro, M Kuleva. Increased uterine artery pulsatility index at 34 weeks and outcome of pregnancy. Ultrasound Obstet Gynecol 2011. [Google Scholar]
- AA Baschat, U Gembruch, CR Harman. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol 2001. [Google Scholar]
- J Unterscheider, S Daly, MP Geary, MM Kennelly, FM McAuliffe, K O'Donoghue. Predictable progressive Doppler deterioration in IUGR: does it really exist?. Am J Obstet Gynecol 2013. [Google Scholar]
- E Ferrazzi, M Bozzo, S Rigano, M Bellotti, A Morabito, G Pardi. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth-restricted fetus. Ultrasound Obstet Gynecol 2002. [Google Scholar]
- HB Westergaard, J Langhoff-Roos, G Lingman, K Marsal, S Kreiner. A critical appraisal of the use of umbilical artery Doppler ultrasound in high-risk pregnancies: use of meta-analyses in evidence-based obstetrics. Ultrasound Obstet Gynecol 2001. [Google Scholar]
- L Rai, S Lekshmi. Value of third trimester uterine artery Doppler in high-risk pregnancies for prediction of adverse perinatal outcome. J South Asian Fed Obstet Gynecol 2010. [Google Scholar]
- P Shwarzman, AY Waintraub, M Frieger, A Bashiri, M Mazor, R Hershkovitz. Third-trimester abnormal uterine artery Doppler findings are associated with adverse pregnancy outcomes. J Ultrasound Med 2013. [Google Scholar]
- Introduction
- Materials and Methods
- Results
- Obstetric and clinical details
- Doppler examination details of the study population
- Uterine artery PI
- Middle cerebral artery
- Ductus venosus doppler and umbilical vein pulsations
- Progression pattern for the doppler abnormalities in the study population
- Adverse perinatal outcomes
- Discussion
- Conclusion
- Source of Funding
- Conflict of Interest
