- Visibility 181 Views
- Downloads 11 Downloads
- DOI 10.18231/j.ijogr.2023.049
-
CrossMark
- Citation
Primary ovarian insufficiency- an overview: Part 1 definition, aetiology, clinical relevance
Introduction
Menopause typically occurs between the ages of 51.4 and 55.2, however some women experience it as early as 40, at which point it is diagnosed as premature ovarian failure (POF). To underline that the fundamental deficit was the ovarian function rather than the central failure in gonadotrophin production from the pituitary, Fuller Albright dubbed the disease primary ovarian insufficiency (POI) in 1942.[1] Primary ovarian failure (POF) was a more common term before Cooper and colleagues (Nelson, 2009; Cooper, et al., 2011) reintroduce the term POI in the American consensus meeting as “insufficiency” more accurately describes the fluctuating nature of the condition, and does not carry the negative connotation of “failure” and the term “primary” to denote it is originating in ovary.[2] Secondary ovarian insufficiency originates from a central defect in gonadotropin secretion by the pituitary. The term POF suggests that the state of ovarian failure is irreversible which is not strictly true. The term “premature menopause” and POF both are inaccurate as many patients intermittently produce estrogen and ovulate and few may experience the intermittent return of regular menstruation 5 to 10% of women can conceive and have a normal pregnancy and interestingly this can occur many years after diagnosis. POI is therefore a spectrum of disorders and is a continuum of impaired ovarian function and reduced fecundity and the term “insufficiency” truly reflects the possibility of intermittent resumption of ovarian function (ACOG 2014). POF is a pathological condition that should not be considered a hastening of natural menopause (ACOG 2017). The ovarian follicular function fluctuates in about 50% of women with POF and 5–10% of women with the diagnosis may eventually conceive.[3], [4] Moreover, the term ‘failure’ has a negative connotation and conveys a sense of hopelessness which does not help when trying to counsel a woman and her family sensitively.
Definition
Premature ovarian insufficiency is a clinical syndrome defined by loss of ovarian activity before the age of 40 and is characterized by menstrual disturbance (amenorrhea or oligomenorrhea) with raised gonadotropins and low estradiol. (ESHRE 2016).[5]
Cessation of ovarian function in women aged between 40 and 45 is termed early menopause.[5]
Prevalence
There are not many studies to find out the prevalence of POI and most are from old literature. The longitudinal cohort study of Coulam and colleagues, which aimed at establishing the age-specific incidence of POI, found that 1% of women entered natural menopause before the age of 40 (Coulam, et al., 1986). The prevalence of early menopause (in the 40 to 44 age group) is ten times higher (Coulam, et al., 1986).[6] Luborsky and colleagues reported a 1.1% prevalence of POI in a cross-sectional survey of women aged 40-55 years (Luborsky, et al., 2003).[7]
|
Age |
Prevalence of POI |
|
By 40 years |
1 in 100 |
|
By 30 years |
1 in 1000 |
|
By 20 years |
1 in 10000 |
|
By 15 years (as primary amenorrhea) |
1 in 100000 |
Prevalence of POI is greatly influenced by ethnicity, lifestyle, genetics, and familial and socio-economic factors, Luborsky found that the prevalence of POI in the USA was higher in African-American and Hispanic women than in Caucasians, and the prevalence was lower in women with Chinese and Japanese ancestry.[7] Regular smoking can lead to earlier menopause.[8] Those who exercise regularly are likely to attain menopause later.[9] Women with lower IQ and cognitive scores in childhood reached menopause earlier than women with higher IQ scores.[10]
Aetiology
The cause of POF is largely unknown: in the largest clinical series, a definitive etiology was identified among only 44 of 373 women (11.8%) who presented over a 10-year period. [11]
|
Causes |
Conditions |
|
Genetics |
Fragile X syndrome Familial ovarian failure Small X chromosome defect FSH and LH receptor Mutation Galactosemia Blepharophimosis, ptosis, and epicanthus inversus syndrome (BPES) Mutations in BMP15, FOXL2, FMR1, FMR2, SF1, SF2, CYP19A1, etc. |
|
Gonadal dysgenesis |
46, XX gonadal dysgenesis 46, XY gonadal dysgenesis Perrault syndrome Turner syndrome |
|
Autoimmune |
Isolated autoimmune ovarian failure • Addison’s disease • Autoimmune polyendocrine syndromes 1 and 2 • Autoimmune hypothyroidism • Others-coeliac disease, type 1 DM, myasthenia gravis, systemic lupus erythematous, thrombocytopenic purpura, vitiligo, alopecia, pernicious anemia, rheumatoid arthritis, Crohn’s disease, Sjogren’s syndrome, primary biliary cirrhosis |
|
Iatrogenic |
Chemotherapy – especially alkylating agents and dependent on cumulative dose Radiation treatment - cumulative dosage and field size matter Early menopause and/or diminished ovarian reserve have been linked to bilateral oophorectomy and other pelvic surgeries. Oophorectomy, hysterectomy, embolization of the uterine artery, and ovarian cyst and endometriosis surgery on both ovaries are all options. |
|
Infection |
Mumps Rubella EBV |
|
Enzyme disorders |
17 alpha-hydroxylase deficiency 17-20 desmolase deficiency |
Idiopathic POI
Almost 90% of POI are idiopathic[3] but the exact number of women diagnosed with idiopathic POI has not been established, as it is largely dependent on the setting, patient population, and the available investigations for identifying causation. As our knowledge grows on POI it is more likely that the number of idiopathic cases will reduce. “In a significant number of women with POI, the cause is not identified and these women are described as having unexplained or idiopathic POI”.[12] (ESHRE 2016)
Environmental POI
Smoking, alcohol, nutrition, and exposure to endocrine disruptors are implicated as influencing the age of menopause, but are not readily diagnosable causes of POI. It is recommended that women at risk of POI discontinue smoking.[12] Some observational studies have revealed that smoking may cause early menopause in women.
Iatrogenic POI
Radiotherapy and chemotherapy can lead to POI. Alkylating agents are gonadotoxic in childhood as well in adulthood.[13] The gonadotoxic effect of chemotherapy is largely drug-and dose-dependent and is related to age. There are studies indicating a relation between early menopause in women who had undergone a hysterectomy and in women who had undergone tubal sterilization compared with women without surgery.[14]
Infectious POI
Many viruses like mumps, rubella, EBV, HIV, herpes zoster, cytomegalovirus, tuberculosis, malaria, varicella, and shigella can harbor in the ovary but only mumps oophoritis has been considered a cause of POI, explaining 3-7% of POI cases.[15] There is no indication for infection screening in women with POI.
Autoimmune POI
POI is more common in women with specific autoimmune illnesses, and POI is more common in POI than in the general population. Approximately 25% of individuals with isolated spontaneous POI are positive for autoimmune markers but there is poor sensitivity and/or specificity of the common autoantibodies.
The most clinically important association is with autoimmune Addison’s disease, in the context of the autoimmune polyendocrine syndrome (APS). POI of adrenal autoimmune origin is the most frequent type observed in 60 to 80% of patients with autoimmune POI.[16], [17]
|
Addison's disease |
APS type 1 |
APS type 2 |
|
|
APS-1 is an AR disease caused by a mutation in the AIRE gene presenting mainly in children with candidiasis, hypoparathyroidism, and Addison’s disease. Ovarian insufficiency occurs in 15% of cases. |
APS-2 is probably polygenic with an AD pattern of inheritance which comprises Addison's disease, autoimmune thyroid diseases, and/or type I diabetes with an array of less common organ-specific autoimmune conditions (e.g. coeliac disease). Ovarian insufficiency occurs in approximately 10% of cases. |
|
Conditions associated |
||
|
Thyroid disease, coeliac disease, pernicious anemia, type 1 diabetes mellitus, gonadal failure, vitiligo, alopecia, myasthenia gravis |
Primary: Addison's disease, hypoparathyroidism, chronic candidiasis (mucocutaneous) Other: gonadal failure, hepatitis, atrophic gastritis, pernicious anemia, malabsorption, alopecia, type 1 diabetes, autoimmune thyroid disease, diabetes insipidus, hypopituitarism |
Primary: Addison's disease, type I diabetes mellitus, autoimmune thyroid disease Other: gonadal failure, vitiligo, pernicious anemia, alopecia, myasthenia gravis, arthritis (seronegative/rheumatoid), diabetes insipidus |
At present, only the detection of SCA (steroid-cell autoantibodies) can ensure the accurate diagnosis of POI caused by autoimmune ovarian damage, and ovarian biopsy is not indicated for the diagnosis of autoimmune POI as in the absence of autoantibodies, no infiltration of ovaries by immune cells has been documented. Major autoantigens recognized by SCA are 17α-hydroxylase (17α-OH) and cytochrome P450 side-chain cleavage enzyme (P450SCC).[18] In women with Addison's illness, circulating SCA may indicate a high chance of POI years before clinical diagnosis. ACA and 21-hydroxylase autoantibodies (21OH-Ab) are the most sensitive markers for autoimmune POI. In nearly 90% of instances, cryo-sections of ovaries with peripheral 21OH-Ab show SCA, 17α-hydroxylase antibodies, and/or P450SCC antibodies. Without 21OH-Ab, fewer than 0.5 percent of POI patients will test positive for SCA, 17α-OH-Ab, or P450SCC-Ab, which is similar to the general population.[19] The predictive value of a commercially available serum anti-ovarian antibody test (an indirect immunofluorescence assay using cynomolgus monkey ovary) to identify these women is poor. ESHRE 2016 recommended that adrenocortical antibodies (ACA) and/ or 21OH-Ab should be measured in every POI patient because of the possibility of subclinical or latent Addison’s disease in POI patients and POI patients with a positive 21OH-Ab/ACA test should be referred to an endocrinologist. In women negative for 21OH-Ab/ACA, there is no indication to analyse these autoantibodies in the future, in the absence of clinical signs or symptoms suggestive of adrenal insufficiency.[20]
POI is associated most commonly with thyroid autoimmunity (14–27%) when adrenal autoimmunity is absent. At present, the most sensitive assay to detect an autoimmune thyroid process is the analysis of TPO-Ab. ESHRE 2016 recommended screening for thyroid antibodies (TPO-Ab) should be performed in women with POI. In patients with a positive TPO-Ab test, thyroid-stimulating hormone (TSH) should be measured every year and a referral to an endocrinologist should be considered. In the case TPO-Ab is negative, in the absence of clinical signs or symptoms of hypo- or hyperthyroidism, follow-up of thyroid function should be applied to the general population of women.
The prevalence of undetected diabetes in POI is 2.5%[21] and it is thought Type 1 DM patients are prone to POI. At present insufficient evidence to recommend routine screening of POI women for diabetes. (ESHRE 2016)
The diagnosis of autoimmune oophoritis can be made based on:
Clinical evidence of primary ovarian insufficiency (POI), including menstrual dysfunction, low estradiol, and high serum gonadotropin concentrations.
The presence of steroidogenic cell autoantibodies.
The exclusion of other causes of POI such as X chromosome defects and the FMR1premutation.
Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in a woman with proven spontaneous POI. Autoimmune thyroid illness does not always mean the POI is autoimmune. Anti-21-hydroxylase antibodies by immunoprecipitation test or indirect immunofluorescence utilising adrenal tissue as substrate are the only approved markers for autoimmune oophoritis.
Ovarian biopsy for autoimmune oophoritis is no longer advised. Autoimmune oophoritis ovarian biopsies with lymphocytic infiltration of secondary and antral but not primordial follicles. Resistant ovary syndrome is clinically similar to POI, although the ovary has normal ovarian reserve for its age owing to receptor block from medications, antibodies to gonadotrophins, or post-receptor abnormalities.
|
1. |
Autoimmune oophoritis is one of the known causes of primary ovarian insufficiency (POI, commonly referred to as premature ovarian failure) and is found in approximately 4 percent of women who present with spontaneous POI. |
|
2. |
It may occur as part of type I and type II syndromes of polyglandular autoimmune failure, which are associated with autoantibodies to multiple endocrine and other organs. |
|
3. |
All women who present with spontaneous POI be tested for 21-hydroxylase antibodies by immunoprecipitation or antiadrenal antibodies by indirect immunofluorescence to identify those women who have autoimmune oophoritis. The predictive value of a commercially available serum anti-ovarian antibody test (an indirect immunofluorescence assay using cynomolgus monkey ovary) has an unacceptably high false-positive rate, so it is not recommended use. |
|
4. |
The serum antiadrenal and/or anti-21-hydroxylase antibodies, which are measured to diagnose autoimmune oophoritis, will also identify patients with asymptomatic adrenal insufficiency (eg, testing for antiadrenal and anti-21-hydroxylase antibodies may serve the dual purpose of making the diagnosis of autoimmune oophoritis and screening for autoimmune adrenal insufficiency). Those with positive antibodies should be carefully evaluated for the presence of adrenal insufficiency. |
|
5. |
It is not recommended to take an ovarian biopsy for diagnosis of autoimmune oophoritis. |
|
6. |
Women should also be screened for autoimmune thyroid disease with thyrotropin (TSH), thyroxine (T4), and antithyroid peroxidase antibodies. |
|
7. |
Management of women with autoimmune POI is the same as that of women with other causes of POI. Estrogen replacement is necessary to prevent bone loss and other long-term consequences. |
Chromosomal POI
10-12% of women diagnosed with POI have chromosomal abnormalities, of which the majority (94%) are X chromosomal abnormalities (X structural abnormalities or X aneuploidy).[21] In the presence of a Y chromosome, there is an elevated risk of developing gonadal neoplasia (45%).[22] Every woman with a Y chromosome, whether or not she has an SRY gene mutation, should be counseled about the risk of development of a gonadal tumor and gonadectomy should be advised. ESHRE 2016 recommended that all women with POI not caused by medical intervention should have chromosomal study done.
In Turner syndrome, there is accelerated atresia of the primordial follicles during later fetal life. Subsequently, the ovarian follicle pool is depleted by the time of puberty among those with the 45 XO genotype and they present with primary amenorrhoea. Among those with a mosaic genotype such as 45XO/46XX (when the X chromosome is missing in some cells but not in others), the rate of atresia, although accelerated, is relatively slower and these women present with secondary amenorrhoea.
Another X chromosome disorder in which POF is well described is fragile X syndrome premutation, where the prevalence of POF is 13–26%.[23] Unlike the classical fragile X syndrome, where the FMR-1 gene contains >200 repeats of the CGG codon, in premutation the individual is phenotypically normal and there are 55–200 repeats. The mechanisms by which FMR-1 premutation causes POF are not clearly understood. One hypothesis is that the increase in FMR-1 transcription results in excess RNA protein clusters, causing premature degradation of ovarian follicles.[24] Most cases of the syndrome result from the expansion of a CGG trinucleotide repeat located in the 5′ UTR of the FMR1 gene to more than 200 repeats. Alleles of FMR1 that include this extended repeat are known as the complete mutation. Locus-specific hypermethylation and chromatin remodelling due to the expanded repetition silence the FMR1 gene epigenetically. Many premutation alleles (55–199 CGG repeats) are unstable and at risk for complete mutation expansions in one generation. 94% of alleles with 90+ repetitions get fully mutated. Expansion to a complete mutation usually always happens from mother to kid.
Fragile-X testing is indicated in all women with POI, not only to establish the causation of POI but also because the presence of the mutation could have major implications for herself and her family. Family members may be carriers too and therefore have a risk of developing POI and a risk of having (grand) children with the fragile-X syndrome. In addition, the patient herself may already have daughters, who may be carriers. This requires careful counselling before the test is performed, including permission from the patient to perform the test.
For this reason, in some countries, women who wish to avoid the risk of having a child affected with FXS are offered preconception genetic screening for FMR1 premutation. Currently, FMR1 premutation carriers who wish to conceive and avoid the risk of having an affected child have three options:
To conceive using In Vitro Fertilization and preimplantation genetic testing for monogenic gene diseases (IVF-PGT-M).
II. Spontaneous conception and prenatal genetic diagnosis during pregnancy by either chorionic villous sampling (CVS) or amniocentesis (AC).
Using a donor oocyte.
Spontaneous conception carries a risk of bearing an affected child and the need to perform a termination of pregnancy. Termination of pregnancy involves medical, emotional, and ethical issues. On the other hand, IVF using PGT-M avoids the need for a termination of pregnancy and offers the opportunity to transfer only non-carrier embryos. However, this procedure arouses financial and emotional difficulties and is not the obvious choice for a fertile couple, especially considering the higher prevalence of ovarian dysfunction and reduced ovarian response observed in FMR1 premutation carriers undergoing IVF compared to non-carrier women. For women who are known cases of POI due to this condition or daughters of a mother with FMR1 premutation, we can take advantage of the latest developments in the rapidly evolving field of fertility preservation.
An additional reason to counsel about Fra-X testing is the risk of fragile-X-associated tremor/ataxia syndrome (FXTAS), a late-onset neurological problem predominantly in male carriers of the Fra-X-mutation.
Galactosaemia is associated with a higher risk of developing POI. In galactosemia, mechanisms involved in the pathogenesis of POF include direct toxicity from increased metabolites (galactose-1-phosphate and galactitol); aberrant glycosylation of glycoproteins, and glycolipids involved in ovarian function; and activation of apoptosis of oocytes and ovarian stromal cells.[25]
Autosomal genetic testing is not at present indicated in women with POI except in POI patients presenting with typical phenotype characteristics of a specific gene mutation.[26]
Early Ovarian Aging vs. POI
Nikolaou and Templeton postulated early ovarian ageing in 2003. 10% of women have EOA. Early ovarian age may cause asymptomatic fertility, monthly irregularity, and early menopause. EOA is asymptomatic and precedes early menopause or premature ovarian insufficiency (POI). The EOA hypothesis suggests that EOA follows a similar model of follicle loss to normal ovarian aging, with relatively fixed reproductive milestones.[27] However, the onset of the ovarian aging process occurs significantly earlier. Women with EOA may have accelerated follicle depletion compared with women with normal ovarian ageing. In women with fertility problems who have a low ovarian reserve, AFC showed a steeper age-related decline compared with egg donors and a shorter end of fertility to menopause interval is seen in women with POI (a median of 4 years compared with 10 years). The EOA hypothesis accepted the previous suggestion of Faddy et al.[28] that the rate of decline of the ovarian reserve depends mainly on the size of the remaining pool of follicles and accelerates when the number of remaining follicles falls below a critical level of around 25 000. However, with no longitudinal histological studies in women with EOA or POI, it is not completely understood whether the early onset of ovarian aging is due to a smaller follicle pool at birth or to an increased rate of loss.[29]
|
|
Early ovarian Aging |
POI |
|
Prevalence |
10% |
1% |
|
Menstruation |
Regular |
Oligo/ amenorrhoea |
|
Symptoms |
Asymptomatic |
Symptoms of estrogen deficiency |
|
FSH |
FSH is normal or mildly elevated |
FSH markedly elevated (>25–30 IU/l) |
|
Estrogen replacement |
Not indicated |
Recommended |
|
Fertility |
Fertility is preserved until late |
Spontaneous conception ~5% |
Taken from Maclaran K, Nikolaou D. Early ovarian aging. The Obstetrician & Gynaecologist. 2019; 21:107–16.[29]
Clinical Features
Elicitation of a detailed history would suggest the diagnosis of POF by excluding other common causes of amenorrhoea. These include pregnancy, hypothalamic amenorrhoea (caused by anorexia nervosa, exercise, stress, and weight loss), hyperprolactinemia (symptoms include headaches and galactorrhoea), and polycystic ovary syndrome (symptoms include hirsutism and acne). History of chemotherapy, radiotherapy, or pelvic surgery in the past is relevant. A history of obstetric catastrophe, severe bleeding, dilatation, and curettage or infection indicates a uterine cause (Asherman syndrome). History pertinent to autoimmune disorders, including hypothyroidism or adrenal insufficiency, should also be elicited. A family history of POF can be recorded in 14–31% of cases and a definite familial disorder can be identified in some of these. Perrault syndrome (XX gonadal dysgenesis with sensorineural deafness), FSH receptor mutations, and fragile X permutations are relatively more common among women with a familial history of POF.[30]
A physical examination, including height, weight, and body mass index, is essential and the recognition of any dysmorphic features suggesting a chromosomal or genetic cause of POF is important. Turner syndrome patient will have short stature, low hairline, shielded chest, increased carrying angle, etc. IQ and cognitive function should be checked. A breast examination is indicated if there is any evidence of galactorrhoea. History of intellectual disability, tremor, and ataxia in family points towards premutation of FMR1 gene.
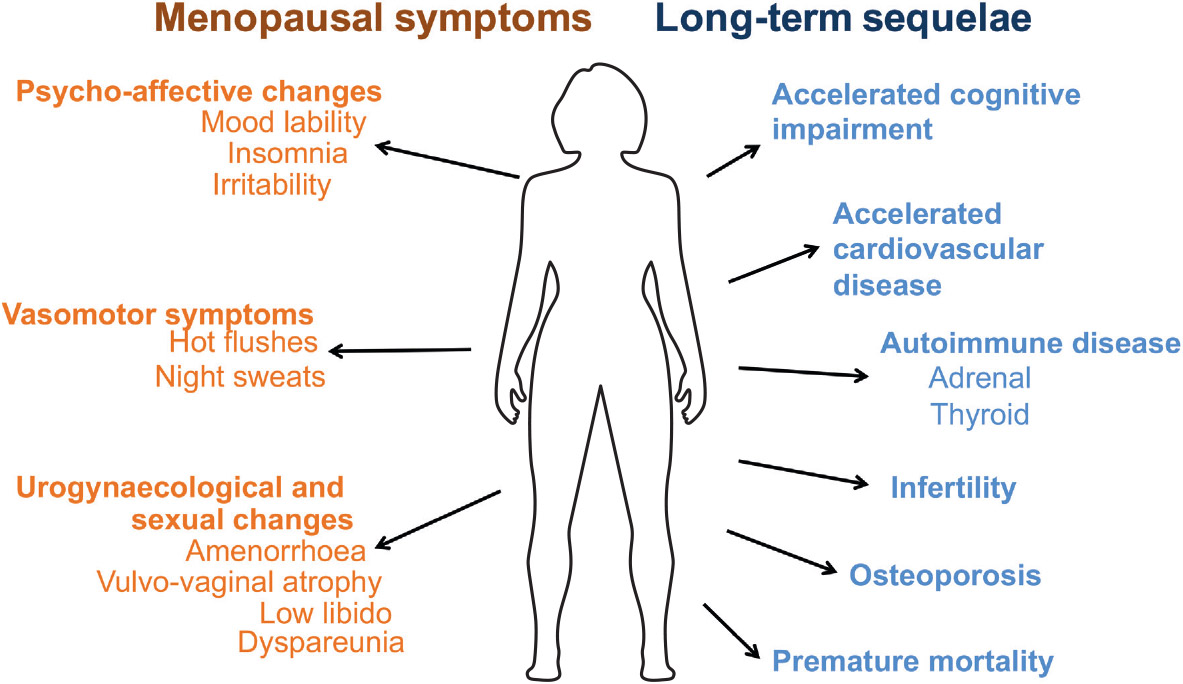
Physical and emotional symptoms include hot flashes, night sweats, dry skin, vaginal dryness, irregular or absent menstruation, anxiety, depression, mental fog, irritability, nervousness, decreased libido, and increased autoimmune disruption.
Menstrual Disturbances: Irregularity of the menstrual cycle in the form of amenorrhoea or oligomenorrhoea is the main presenting feature. In the majority of cases, amenorrhoea is secondary and follows normal pubertal development. In 20% of cases, amenorrhoea may be primary and pubertal development may be delayed. Occasionally the pattern of menstrual irregularity presents with failure of menses to return after cessation of hormonal contraception. Among females with primary ovarian insufficiency and a normal karyotype, 6% have a premutation in the FMR1 gene. Although the onset of menstruation appears to be normal among premutation carriers in adolescence, approximately 1% of premutation carriers will experience their final menses before age 18 years.[30]
The most common urogenital symptoms include vaginal dryness, vaginal irritation, and itching. Urogenital atrophy and hypoestrogenism interferes also with sexual functioning.
Patients with POI are threatened by a decrease in bone mineral density (BMD). Numerous studies have revealed a significant decrease in BMD in POI patients. Uygur et al. found that both the femoral neck bone and spinal bone BMD were significantly lower in POI patients than in measurements of the control group. POI women are at risk for cardiovascular disease because to endothelial dysfunction, autonomic dysfunction, altered lipid profiles, insulin action abnormalities, and metabolic syndrome. Global cognition and verbal memory scores are linked to POI neurological illness risk. In genetic POI, cognitive abnormalities may be inherited rather than environmental. Cognitive deterioration after chemotherapy or GnRH analogues is inconsistent.
|
History |
Examination |
|
1. Age at menarche 2. Maternal age at menopause 3. Frequency and duration of previous menstrual cycles 4. History of tobacco use 5. Prior surgical procedures or exposure to gonadotoxic chemicals 6. Premature ovarian failure in first- or second-degree relatives 7. Male ancestors with a history of mental impairment 8 Do you have a family history of any genetic disorders? 9. Personal or family history of an autoimmune condition |
1. Height of the patient 2. Weight of the patient 3. Secondary sexual characteristics 4. Skin pigmentation- vitiligo (autoimmune)/ hyperpigmentation (autoimmune adrenal insufficiency) 5. Dysmorphic features (to rule out other syndromic associations). Ophthalmologic examination (to look for blepharophimosis /ptosis) 6. Neck swelling (to rule out goiter [autoimmune etiology]) 7. In-person genital checkup (to look for atrophic changes) |
Long term Sequelae of POI
Cardiovascular Disease: Impaired endothelial function due to lack of estrogen is the main culprit behind shortened life expectancy of patients with untreated POI.
Cognitive impairment and psychological effect: Increased risk of cognitive impairment or dementia has been seen in women who undergo oophorectomy (unilateral/bilateral) before the onset of menopause and also seen in women with POI and also at increased lifetime risk of major depression and anxiety.
Subfertility: Women with POI have very poor ovarian reserve and often require the help of ART.
Diminished sexual well-being: Women with POI have poor sexual performance due to pain and vaginal dryness because of hypoestrogenism and androgen deficiency is the cause of low libido, poor genital arousal, and orgasm, as well as blunted motivation and diminished wellbeing.
Osteoporosis and osteopenia: Due to a lack of estrogen there is an increase in bone remodeling and bone resorption exceeds bone formation and leading to reduced bone mineral density. Women with POI are particularly prone to lumbar vertebral fracture.
Diagnosis and management part is discussed in part 2 of this article.
References
- F Albright, P Smith, R Fraser. A syndrome characterized by primary ovarian insufficiency and decreased stature. Am J Med Sci 1942. [Google Scholar]
- AR Cooper, VL Baker, EW Sterling, ME Ryan, TK Woodruff, LM Nelson. The time is now for a new approach to primary ovarian insufficiency. Fertil Steril 2011. [Google Scholar]
- LM Nelson, JN Anastasi, LM Kimzey, RA Defensor, KJ Lipetz, BJ White. Development of luteinized graafian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J Clin Endocrinol Metab 1994. [Google Scholar]
- YMV Kasteren, J Schoemaker. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update 1999. [Google Scholar]
- L Webber, M Davies, R Anderson, J Bartlett, D Braat, B Cartwright. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 2016. [Google Scholar]
- CB Coulam, SC Adamson, JF Annegers. Incidence of premature ovarian failure. Obstet Gynecol 1986. [Google Scholar]
- JL Luborsky, P Meyer, MF Sowers, EB Gold, N Santoro. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod 2003. [Google Scholar]
- EB Gold, SL Crawford, NE Avis, CJ Crandall, LE Waetjen, JS Lee. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol 2013. [Google Scholar]
- T Dorjgochoo, A Kallianpur, YT Gao, H Cai, G Yang, H Li. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women's Health Study. Menopause 2008. [Google Scholar]
- D Kuh, S Butterworth, H Kok, M Richards, R Hardy, ME Wadsworth. Childhood cognitive ability and age at menopause: evidence from two cohort studies. Menopause 2005. [Google Scholar]
- A Bachelot, A Rouxel, N Massin, J Dulon, C Courtillot, C Matuchansky. Phenotyping and genetic studies of 357 consecutive patients presenting with premature ovarian failure. Eur J Endocrinol 2009. [Google Scholar]
- L Webber, M Davies, R Anderson, J Bartlett, D Braat, B Cartwright. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 2016. [Google Scholar]
- C Decanter, F Morschhauser, P Pigny, C Lefebvre, C Gallo, D Dewailly. Anti-Mullerian hormone follow-up in young women treated by chemotherapy for lymphoma: preliminary results. Reprod Biomed Online 2010. [Google Scholar]
- N Visvanathan, G Wyshak. Tubal ligation, menstrual changes, and menopausal symptoms. J Womens Health Gend Based Med 2000. [Google Scholar]
- A Kokcu. Premature ovarian failure from current perspective. Gynecol Endocrinol 2010. [Google Scholar]
- M Dittmar, GJ Kahaly. Polyglandular autoimmune syndromes: immunogenetics and long-term follow-up. J Clin Endocrinol Metab 2003. [Google Scholar]
- CA Silva, LY Yamakami, NE Aikawa, DB Araujo, JF Carvalho, E Bonfa. Autoimmune primary ovarian insufficiency. Autoimmun Rev 2014. [Google Scholar]
- S Chen, J Sawicka, C Betterle, M Powell, L Prentice, M Volpato. Autoantibodies to steroidogenic enzymes in autoimmune polyglandular syndrome, Addison's disease, and premature ovarian failure. J Clin Endocrinol Metab 1996. [Google Scholar]
- VK Bakalov, VH Vanderhoof, CA Bondy, LM Nelson. Adrenal antibodies detect asymptomatic auto-immune adrenal insufficiency in young women with spontaneous premature ovarian failure. Hum Reprod 2002. [Google Scholar]
- M Davies, R Anderson, J Bartlett, D Braat, B Cartwright, R Cifkova. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 2016. [Google Scholar]
- H Kalantari, T Madani, SZ Moradi,, Z Mansouri, N Almadani, H Gourabi. Cytogenetic analysis of 179 Iranian women with premature ovarian failure. Gynecol Endocrinol 2013. [Google Scholar]
- L Michala, D Goswami, SM Creighton, GS Conway. Swyer syndrome: presentation and outcomes. BJOG 2008. [Google Scholar]
- MD Wittenberger, RJ Hagerman, SL Sherman, A Mcconkie-Rosell, CK Welt, RW Rebar. The FMR1 premutation and reproduction. Fertil Steril 2007. [Google Scholar]
- KL Bretherick, MR Fluker, WP Robinson. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet 2005. [Google Scholar]
- T Forges, P Monnier-Barbarino, B Leheup, P Jouvet. Pathophysiology of impaired ovarian function in galactosaemia. Hum Reprod Update 2006. [Google Scholar]
- ID Tonkelaar, ER teVelde, CW Looman. Menstrual cycle length preceding menopause in relation to age at menopause. Maturitas 1998. [Google Scholar]
- W Vegetti, MG Tibiletti, G Testa, L Yankowski, F Alagna, E Castoldi. Inheritance in idiopathic premature ovarian failure: analysis of 71 cases. Hum Reprod 1998. [Google Scholar]
- MJ Faddy, RG Gosden, A Gougeon, SJ Richardson, JF Nelson. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod 1992. [Google Scholar]
- K Maclaran, D Nikolaou. Early ovarian aging. Obstet Gynaecol 2019. [Google Scholar]
- P Arora, DW Polson. Diagnosis and management of premature ovarian failure. Obstet Gynaecol 2011. [Google Scholar]
