- Visibility 64 Views
- Downloads 2 Downloads
- DOI 10.18231/j.ijogr.2023.055
-
CrossMark
- Citation
Critical analysis of misoprostol versus mifepristone with misoprostol in medical termination of pregnancy
- Author Details:
-
Hanumant V Nipanal *
-
Swetha Doddiah
-
Soubhagya R Talawar
Introduction
Termination of pregnancy by any method before the viable period is called abortion. Though medical and surgical methods are methods of abortion, nowadays medical abortion is gaining popularity. Because of its effectiveness as well as technical related benefits.
Unplanned pregnancies are one of the major problems with which patient present to hospital. Most of the patients present in the first trimester and they are managed by the medical method of abortion. But few patients require to undergo second trimester abortion for indications such as foetal anomalies, missed abortion, unwanted pregnancy which was missed in the first trimester, victim of rape. On other side newer diagnostic modalities like ultrasonography, amniocentesis and cordocentesis are detecting more and more anomalies or defects in the growing foetuses. Hence, there is increase in number of medical termination of cases too.
Around 56 million abortions occurring every year worldwide. In eastern part of Asia itself contributing nearly 13 million.[1] As per reports there are 10 mortalities occurring in India in everyday due to unsafe abortions.[2]
In India medical termination of pregnancy can be done up to twenty weeks of pregnancy, now it is increased to 24 weeks as per new amendment of MTP act 2021 under certain specified indications like risk to the life of the pregnant woman or of grave injury of physical or mental health, anomalous foetus, rape or failure of contraception.
Second trimester abortions have more morbidity than first trimester abortions. Incomplete abortion and method failure are the main problems associated with late termination.
Surgical abortion has many disadvantages like perforation of uterus, haemorrhage, sepsis and increased morbidity. The advantages of medical abortions are improvement in abortion access with safety. It also needs less infrastrure.[3], [4], [5], [6]
The WHO has approved combination of mifepristone with misoprostol for abortion up to 9 weeks of gestation.[7] Misoprostol alone has been used for second trimester medical termination of pregnancy with success rate of 97.2% and the mean induction to abortion interval found to be 19.6 hours.[8]
Many researchers had quoted that combination of mifepristone and misoprostol will give better results than misoprostol alone. But with varying results and study done in first and second trimesters separately.
Aims and Objectives
To compare the efficacy of Misoprostol versus combination of mifepristone with Misoprostol in Medical Termination of Pregnancy.
To asses the side effects of Misoprostol and combinationion of Mifepristone and Misoprostol
Material and Methods
The study was undertaken in Obstetrics and Gynaecology department of Gadag Institute of Medical Sciences, Gadag, and Karnataka, India. It is interventional, comparative study conducted from January 2021 to June 2022.
The total number of subjects were 122 of which divided into two groups with 61 study subjects in each. It is calculated by standard formula of proportion using previous study of Meetali Mukund et al. With 95% percent confidence interval, 5% level of significance with 4% desired precision and also applying 10% rule. We use Purposive sampling technique.
Data collection method
Women coming to hospital seeking abortion upto 20 weeks will be admitted in the hospital. Detailed history and detailed examination was done in all the subjects. Written informed consent was taken. Clinical profile of the patient will be taken. Investigations will be done as per department protocol in all subjects. Gestational age will be estimated by last menstrual period date, clinical examination and confirmed by ultrasonography.
Inclusion criteria
All cases those will fulfil indication of Medical Termination of Pregnancy, as per guidelines of MTP Act of 1971.
Exclusion criteria
Previous LSCS
Ectopic pregnancy
Grand multiparous women
Any contraindications to misoprostol as well as mifepristone
Methods of administration of drugs
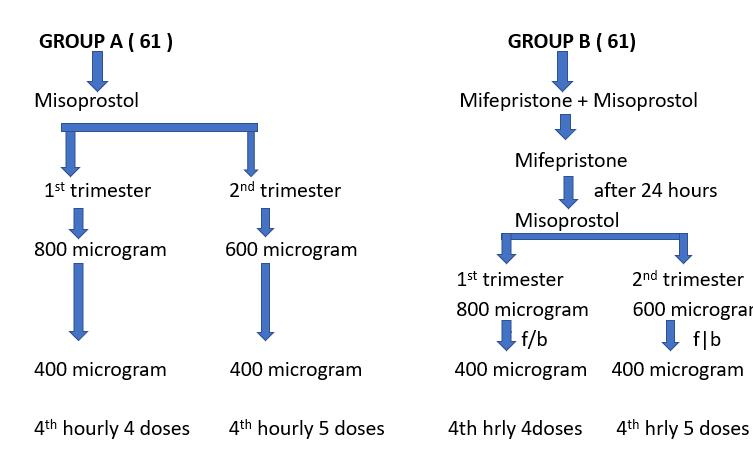
Group A (Misoprostol alone)
Sixty one purposive selected cases will receive tablet misoprostol 600 microgram start placed per vaginally followed by 400 microgram misoprostol tablet placed per vaginally every 4th hourly up to maximum five doses including the first dose in second trimester cases. In case of first trimester cases 800 microgram tablet misoprostol placed per vaginally followed by 400 microgram misoprostol tablet placed per vaginally every 4th hourly up to maximum four doses including first dose.
Group B (Mifepristone and Misoprostol)
Sixty one purposive selected cases will receive tablet mifepristone 200mg orally on empty stomach. After 24 hours will receive tablet misoprostol as mentioned in group A.
The side effects of due drugs was observed and if necessary treatment was given. All the patients received injection Tetanus Toxoid intramuscularly, If not taken after confirmation of pregnancy. If the patient is Rh negative injection anti D 300microgram will be given intramuscularly after abortion. The associated complications like fever, nausea and vomiting was recorded. The patients were monitored with respect to frequency of uterine contractions, pulse rate and blood pressure. Completeness abortion was confirmed by thorough examination of products of conception. If incomplete further steps were taken.
The mean induction abortion interval was considered from administration time of first dose of misoprostol to till complete abortion. The doses analgesics used recorded. The volume of blood loss was measured clinically. The total doses of misoprostol used was calculated. If incomplete abortion cases check curettage was done.
Success: was defined as complete abortion within 24 hours of starting time of misoprostol.
Failure: was defined as incomplete abortion within 24 hours of starting time of misoprostol.
In the events of failure, pre-consent will be taken that termination of pregnancy would be done by surgical method or other medical methods like oxytocin acceleration and managed accordingly.
Statistical method
Data was statistically described in terms of mean (± SD), frequencies and percentages. For Comparison of quantitative variables between the two groups was done using Student T test for independent samples. Chi square test was used for comparing categorical data. P value less than 0.05 was considered as statistical significance. Data analysed by using SPSS software version 22 and t test.
Results
It is a prospective cross-sectional study conducted on 122 subjects of which divided into 2 groups that is group A and group B, each consisting of 61 subjects respectively. In our study Group –A subject’s age ranged from 18 years to 30 years. The majority 27 (44.26%) of them were in the age group of 21 - 25 years and mean age was 25.85 years. In Group –B subjects age ranged from 18 years to 30 years with majority 33 (54.10%) of them were in the age group of 21 -25 years and mean age was 25.05 years. There is no significant age difference between the both groups (p=0.659).
Our study shows uniform distribution between two groups with relation to gravidity (p=0.529). Majority were multigravida consisting of 77 subjects (63.11%). In our study in both groups majority of them were first trimester cases accounting to 74 (60.66%) whereas 48 (39.34%) were second trimester cases.
The mean gestational age in Group A was 9.3 weeks with minimum and maximum was 6.2 weeks and18.4 weeks respectively. On the other side Group B mean gestational age was 12 weeks with minimum and maximum gestational age was 5.3 weeks and 20 weeks respectively.
In our study missed abortion was major indication for MTP in both groups that is group A 43 (70.49%) and group B 38 (62.29%) accounting for 81(66.39%) out of 122 subjects followed by anomalous foetus 25 cases (20.49%) and contraception failure 16 cases (13.12%).
In our study Group A (misoprostol alone) complete abortion was achieved in 48 patients (78.69%) where as in Group B (mifepristone +misoprostol) 57 patients (93.44%) with statically significant value (p=0.001) [Table 1]. Hence, success is more in mifepristone and misoprostol combination.
|
Out come |
Misoprostol group |
Mifepristone +misoprostol group |
Total |
|||
|
Number |
Percentage |
Number |
Percentage |
Number |
Percentage |
|
|
Success |
48 |
78.69 |
57 |
93.44 |
105 |
86.07 |
|
Failure |
13 |
21,31 |
4 |
6.56 |
17 |
1393 |
|
Total |
61 |
100 |
61 |
100 |
122 |
100 |
|
Inferences |
Failure are more in misoprostol group 13 (21.31%) when compared to mifepristone +mifepristone group 4 (6.56%) with statistically significance. (p=0.001) |
In our study the mean induction to abortion interval (IAI) in Group A was 11.803 hours with shortest of 2.5 hours and longest was 22.00 hours. In Group B mean IAI was 5.623 hours with shortest of 2.00 hours and longest was 10.00 hours ([Table 2], [Table 3]).
|
IAI in hours |
Group A |
Group B |
Total |
|||
|
Number |
Percentage |
Number |
Percentage |
Number |
Percentage |
|
|
<12 |
29 |
50.82 |
61 |
93.44 |
88 |
72.13 |
|
12-18 |
21 |
36.07 |
0 |
6.56 |
26 |
23.31 |
|
18-24 |
6 |
13.11 |
0 |
0 |
8 |
6,56 |
|
Total |
61 |
100 |
61 |
100 |
122 |
100 |
|
Mean ± SD |
11.803±4.62 |
5.623±2.833 |
8.713±4.868 |
|
Group |
Mean time of induction to abortion interval (in hours) |
Median |
Standard deviation |
Minimum |
Maximum |
T value |
p value |
|
Group A misoprostol alone |
11.803 |
8.5 |
4.512 |
2.50 |
22.00 |
9.059 |
<0.001 |
|
Group B Mifepristone +Misoprostol |
5.623 |
6.0 |
2.833 |
2.00 |
10.00 |
In our study misoprostol alone group had 34 (55%) patients had side effects, frequent side effect was excessive vaginal bleeding 48%(24) followed by pain abdomen,[6] vomiting,[3] fever[2] and diarrhoea. In misoprostol and mifepristone group 34 (55%) patients had side effects, the most frequent side effect was excessive vaginal bleeding followed by vomiting, pain abdomen and fever. In both the groups 44.26% of the patients have no side effects. Overall the most frequent side effect in both the groups was excessive vaginal bleeding.
The mean doses of misoprostol used in misoprostol alone group was 3.5 whereas mifepristone combination group was 1.8. Which was statistically significant with p value <0.0001.
Surgical intervention was required in 21.31% misoprostol group whereas only 6.55% in mifepristone combination group. The surgical method used was dilatation and curettage.
|
Studies |
Misoprostol group |
Mifepristone + Misoprostol group |
|
Meetali Mukund Khairnar and Abhijeet Madhukar Patil[9] (2018) |
10.82 ± 2hours |
6.22± 2 hours± |
|
Prasanna Lekha Akkenapally[10] (2016) |
10.67±3.96 hours |
6.19±2.70 hours |
|
Nagaria Tripti, Sirmor Namrata[11] (2011) |
12.29± 3.14hours |
6.72±2.26 hours |
|
Priyanka Gupta, Subhaschandra R. Mudanur. Neelamma Patil, et al [12] (2017) |
NA |
9.3 ±hours |
|
Present study |
11.80±4.62 hours |
5.62± 2.83 hours |
|
Authors |
Misoprostol alone |
Mifepristone + misoprostol |
|
Sarita Sonalkar et al[13] (2020) |
NA |
90% |
|
Patil N et al[12] (2017) |
- |
100% |
|
Meetali Mukund et al[9] (2018) |
84.7% |
98.6% |
|
Schreiber et al (2018)[14] |
67.1% |
83.8% |
|
Parul S. Jani et al[15] (2018) |
|
91.2% |
|
Prasanna Lekha Akkenapally[10] (2016) |
89% |
96% |
|
Nagaria T et al[11] (2011) |
90% |
95% |
|
Present study |
78.69% |
93.44% |
Discussion
When Mifepristone is used in abortion it will shorten the induction abortion interval if combined with prostaglandins because it is a progesterone receptor antagonist. The present study aimed to evaluate the combination effects of Misoprostol and Mifepristone in medical method of abortion.
In our study both groups were comparable with respect to patient’s age, period of gestation and parity. The mean age of misoprostol and mifepristone with misoprostol combination group were 25.85 years and 25.05 years respectively. The study conducted by Kapp, Nathalie MD, MPH[16] mifepristone + misoprostol group mean age was 25.7 years and that in misoprostol was 25.5 years. In another study conducted by Justin J Chu et al[17] in 2020, the mean age in misoprostol alone is 32.7 years and in mifepristone + misoprostol is 32.8 years and Jan E Dickinson[18] the mean age was 32 years in both groups.
Around 60.66% of misoprostol and 65.57% of mifepristone and misoprostol group of our study had one prior delivery which comparable which is comparable to study of Jan E Dickinson[18] where misoprostol group had 60.3% and mifepristone with misoprostol group had 57.8% prior one delivery.
In our study the mean period of gestation in misoprostol group was 10.5 weeks whereas in mifepristone + misoprostol group was 12.0 weeks. In a study of Justin J Chu et al[17] in 2020, the mean period of gestation in misoprostol alone group was 13.8 weeks and in mifepristone + misoprostol group was 13.1 weeks.
The mean induction to abortion interval in misoprostol group is 11.803±4.62 hours and in mifepristone +misoprostol group is 5.623±2.833 hours with statistically significant difference with p<0.001 which is comparable with below mentioned previous studies summarized in [Table 4].
RCOG Best practice in comprehensive abortion care[19] 2016 suggests Mifepristone 200 mg orally followed 12-48 hours later by misoprostol 800μgm vaginal then 400μgm every 3 hours till she aborts. As per ACOG recommendation mifepristone 200 mg orally given. After 24-48 hours later misoprostol 800μgm vaginally given. Followed by 400μgm every 3 hours maximum of 5 doses. Whereas WHO suggestion is 200 mg mifepristone given orally. After 36 to 48 hours later misoprostol can be given. FOGSI also suggests 36-48 hours gap after giving mifepristone. But our study results appears that misoprostol can be started after 24 hours later of mifepristone administration which can also give similar results with no serious complications or side effects.
In the present study complete abortion rate was 78.69% in misoprostol alone group where as it was 93.44% in Mifepristone combination group with statically significant value (p<0.001). The same has been compared in [Table 5].
In our study the most frequent side effect in both groups was excessive vaginal bleeding 48-55% where as it is 84.2% in study of Paul S Jani et al.
Conclusion
Medical termination of pregnancy by combination of mifepristone with misoprostol has less induction abortion interval compared to using misoprostol alone. This combination has more success rate and also has lesser side effects. Hence, unnecessary surgical interventions and its associated complications can be avoided.
Source of Funding
None.
Conflict of Interest
None.
References
- . Abortion in Asia. 2018. [Google Scholar]
- . Unsafe Abortions Kill 10 Women Daily in India. 2018. [Google Scholar]
- B Winikoff, C Ellertson, B Elul, I Sivin. Acceptability and feasibility of early pregnancy termination by mifepristone misoprostol; results of a large multicenter trial in the United States. Arch Fam Med 1998. [Google Scholar]
- A Rosenfield. Mifepristone (RU 486) in the United States. What does the future hold?. N Engl J Med 1993. [Google Scholar]
- S Mittal, S Agarwarl, S Kumar, A Batra. Comparison or oral versus vaginal misoprostol & continued use of misoprostol after mifepristone for early medical abortion. Indian J Med Res 2005. [Google Scholar]
- MS Christin, P Bouchard, IM Spitz. Medical termination of pregnancy. N Engl J Med 2000. [Google Scholar]
- M Bhattacharya, SK Das, Gopalakrishnan, BS Kodkany, GG Mukherjee, K Kukherjee. A multicentre randomized comparative clinical trial of 200 mg RU486 (mifepristone) single dose followed by either 5 mg 9-methylene PGE2 Gel (meteneprost) or 600 μg oral PGE1 (misoprostol) for termination of early pregnancy within 28 days of missed menstrual period. Contraception 2000. [Google Scholar]
- D Webster, GC Penney, A Templeton. A comparison of 600 and 200 mg mifepristone prior to second trimester abortion with the prostaglandin misoprostol. Br J Obstet Gynaecol 1996. [Google Scholar]
- PL Akkenapally. A comparative study of misoprostol only and mifepristone plus misoprostol in second trimester termination of pregnancy. J Obstet Gynaecol India 2016. [Google Scholar]
- T Nagaria, N Sirmor. Misoprostol vs Mifepristone and Misoprostol in Second Trimester Termination of Pregnancy. J Obstet Gynaecol India 2011. [Google Scholar]
- MM Khairnar, AM Patil. Study of the Efficacy and Success Rate of Single Dose Oral Mifepristone and Vaginal Misoprostol v/s Vaginal Misoprostol alone for Second Trimester Termination of Pregnancy. MVP J Med Sci 2018. [Google Scholar]
- NG Patil, P Gupta, MD Hittinhalli, SR Mudanur, MK Tehalia, AS Nemagouda. A randomised Controlled Trial to Compare the Efficacy of Preinduction with Mifepristone 12 Hours Versus 24 Hours Prior for Second Trimister Pregnancy Termination. Int J Reprod Contracept Obstet 2017. [Google Scholar]
- S Sonalkar, N Koelper, MD Creinin, JM Atrio, MD Sammel, A Mcallister. Management of early pregnancy loss with mifepristone and misoprostol: clinical predictors of success from a randomized trial. Am J Obstet Gynecol 2020. [Google Scholar]
- CA Schreiber, MD Creinin, J Atrio, S Sonalkar, SJ Ratcliffe, KT Barnhart. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med 2018. [Google Scholar]
- PS Jani. Use of MTP kit (Mifepristone and Misoprostol combination pack) for 1st trimester MTP (up to 63 days) at GMERS Dharpur, Patan, Gujarat, India. Int J Reprod Contracept Obstet Gynecol 2018. [Google Scholar]
- N Kapp, L Borgatta, PG Stubblefield, O Vragovic, N Moreno. Mifepristone in midtrimester medical abortion: a randomized controlled trial. Obstet Gynecol 2007. [Google Scholar]
- JJ Chu, AJ Devall, LE Beeson, P Hardy, V Cheed, Y Sun. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo controlled trial. Lancet 2020. [Google Scholar]
- JE Dickinson, P Brownell, K McGinnis, EA Nathan. Mifepristone and second trimester pregnancy termination for fetal abnormality in Western Australia: Worth the effort. Austr New Zealand J Obstet Gynaecol 2010. [Google Scholar]
- A Dawson, D Bateson, J Estoesta, E Sullivan. Towards comprehensive early abortion service delivery in high income countries: insights for improving universal access to abortion in Australia. BMC Health Serv Res 2016. [Google Scholar]
